Scientific Brief
Advancing an HIV Clinical Program for CCR5 Gene Disruption
Abstract — Gene Editing: Paving the Way for Accelerated Clinical Development of Adoptive Cell Immunotherapies
Precision genome engineering requires technologies that allow efficient and reproducible delivery of DNA, mRNA and RNP-based reagents into a range of primary cells and stem cells. In addition, clinical gene editing requires a transfection platform that is GMP-compliant and scalable to accommodate billions of cells in a single transfection. Here we demonstrate gene deletion in primary cells following transfection of zinc finger nuclease (ZFN) molecules, and we share data on efficient modification of hematopoietic stem and progenitor cells (HSPCs) with high cell viability using the MaxCyte Flow Electroporation® Technology. Finally, the results show long-term engraftment of modified human-derived cells in a mouse model and confirm CCR5 disruption in both peripheral blood mononuclear cells (PBMCs) and bone marrow.
Case Study
High Biallelic CCR5 Gene Disruption Rates Using ZFN mRNA Delivery with Ability for Long-term Engraftment.
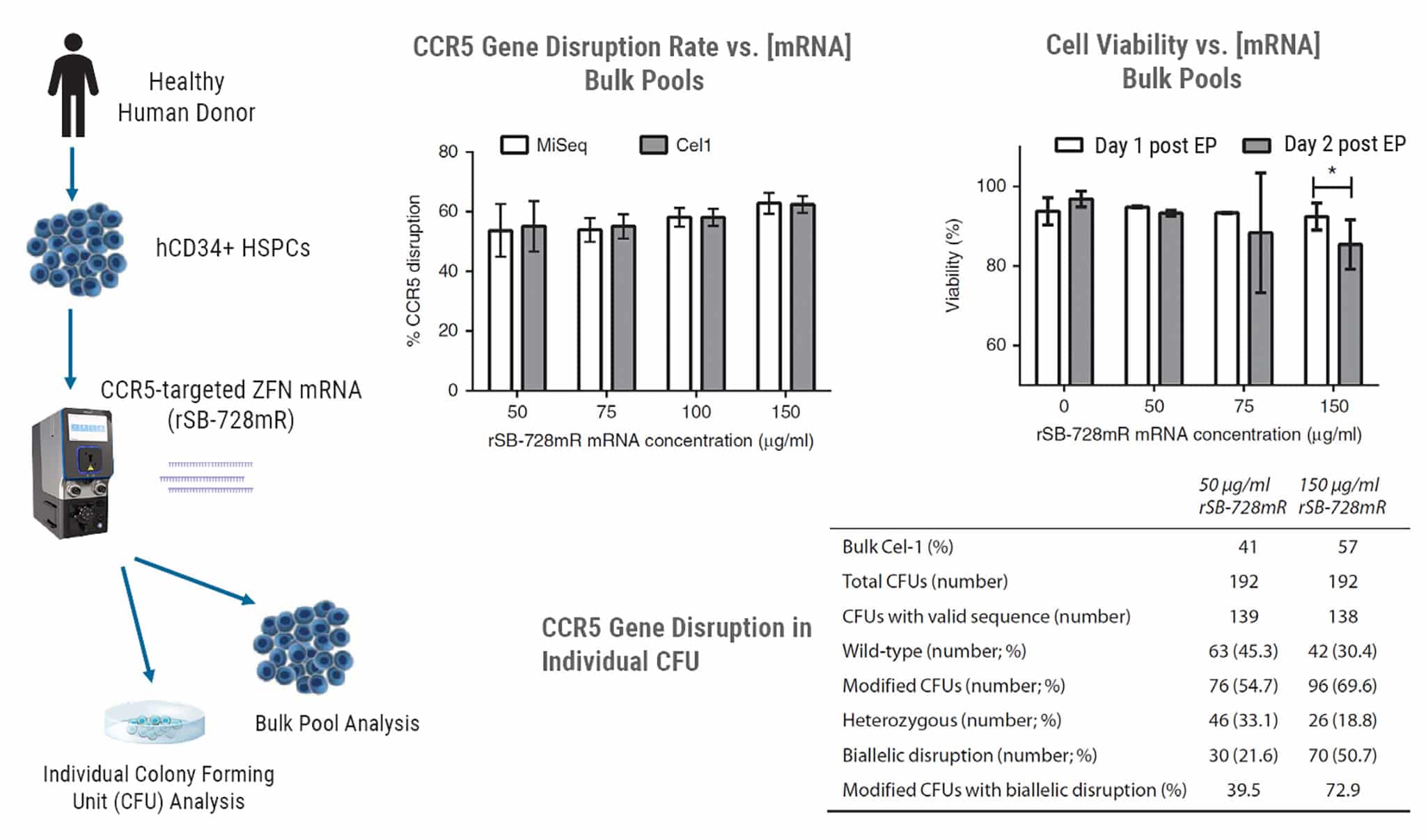
Researchers at Sangamo developed a CCR5- targeted zinc finger nuclease that they showed was active in a variety of CD4 T cells and HSPCs and that conferred resistance to HIV infection. This therapy was advanced to the clinic using adenoviruses to deliver the ZFN constructs. The phase I/II trials showed that CD4 cells with a disrupted CCR5 gene could be engrafted, were safe and persisted. Toxicity related to the adenoviral vector precluded the intended trials from progressing. To rescue the therapy, the company turned to mRNA delivery of the CCR5-specific ZFN using the MaxCyte GTxTM. The work published in Mol Ther. Methods Clin. Dev., 3, 2016 and summarized above demonstrate the rapid progression from process development of ZFN delivery, through manufacturing qualification runs, pre-clinical toxicity studies and initiation of clinical trial NCT02500849 using the MaxCyte GTx. See publication for detailed methods.
Full-scale Engineering Runs Using Different Human Donors & Toxicity Testing

Observations
- No impact on ability for long-term engraftment
- No impact on hematopoietic potential
- No adverse events
- No difference in premature deaths
- No toxicity associated with modified cells
Summary
- Gene editing using MaxCyte® non-viral engineering enables rapid development of next-generation adoptive cell therapies for treatment of a wide variety of diseases.
- The high viability of cells following electroporation allows for high rates of long-term engraftment that are required for patient treatment.
- MaxCyte Flow Electroporation® Technology efficiently (co)delivers a diversity of payloads including mRNA, sgRNA, and RNPs to difficult-to-engineer primary cells commonly used for adoptive cell therapies including hematopoietic stem cells and T cells.
- The high efficiency and low toxicity of MaxCyte Flow Electroporation provides for high levels of nuclease or nickase-mediated gene editing including:
- Gene knockout/disruption
- Gene knock-in
- Single nucleotide gene mutation correction
- MaxCyte clinical scalability and regulatory compliance provide for streamlined clinical translation of new therapies.





