Scientific Poster
Efficient and Scalable T Cell Engineering for Advancing Cancer Immunotherapy of Hepatocellular Carcinoma by a cGMP-Compliant Non-Viral Cell Engineering Platform
Abstract
Chronic hepatitis B virus (HBV) infection can result in hepatocellular carcinoma (HCC), which is the third leading cause of cancer-related deaths worldwide. For autologous immunotherapy of HCC, patient-derived T cells can be engineered to target HBV-specific antigens by exogenous expression of HBV-specific T cell receptors (TCRs). In contrast to viral delivery methods that can be expensive, time-consuming, inefficient, and potentially toxic during treatment due to the long-lived survival of transduced cells, non-viral mRNA electroporation is a transient, cheaper, and potentially safer alternative. However, high efficiency of transfection, high cell viability, and scalability are important parameters for commercial production of quality, clinically-functional engineered cells. Here, we demonstrate seamless optimization of electroporation conditions for efficient and scalable electroporation of activated primary T cells with HBV-specific TCR mRNA at a scale up to 3.9 billion cells. Moreover, T cells could be cryopreserved soon after electroporation and yielded TCR expression and functional cytokine expression comparable to freshly electroporated T cells upon thawing. This study demonstrates a robust and efficient manufacturing process for large-scale, transient TCR-expressing T cells with a cGMP-compliant non-viral cell engineering platform.
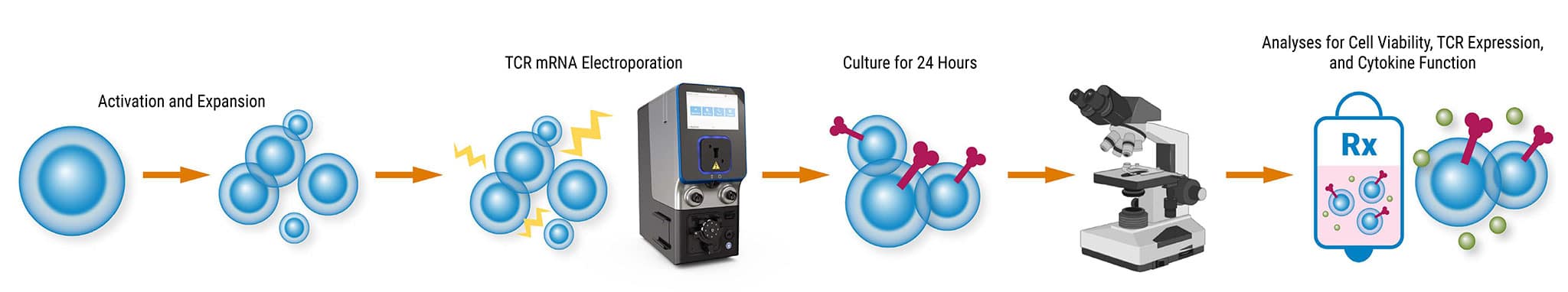
Workflow Schematic
Electroporation Protocol and Cell Density Optimization
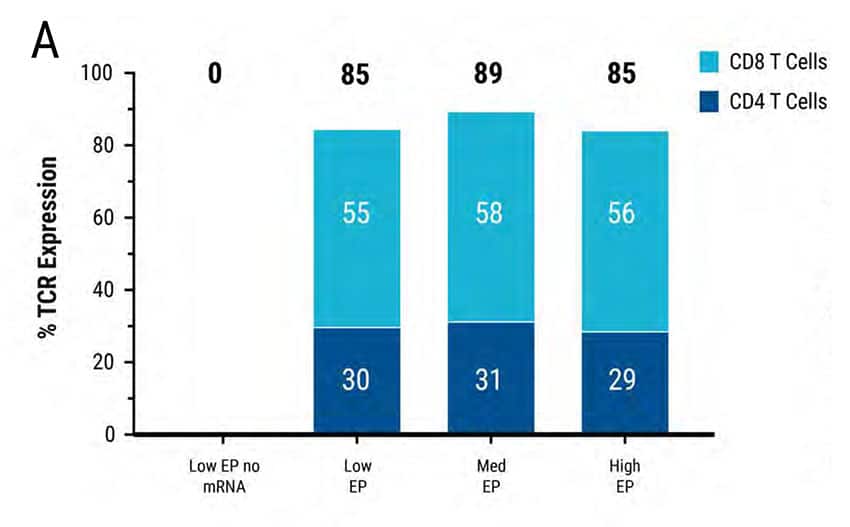
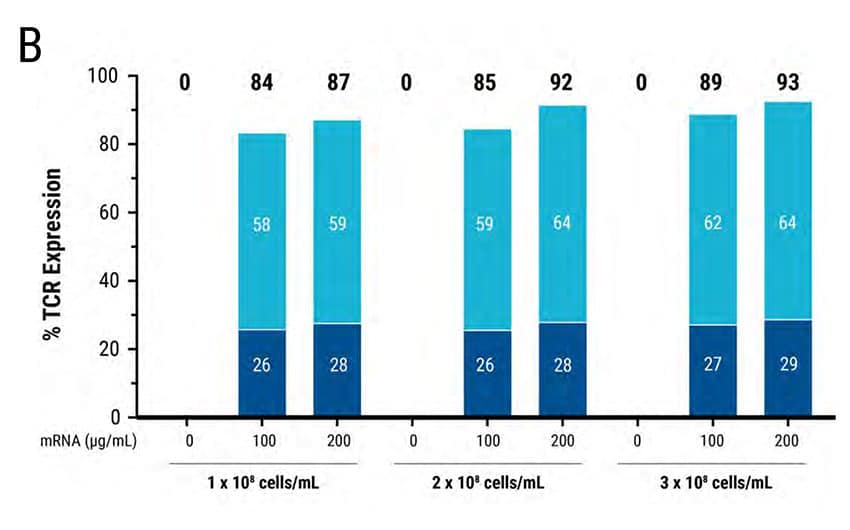
Figure 2: MaxCyte® electroporation enables rapid optimization for mRNA transfection of high-cell-density, primary, activated T cells. 5 x 106 primary activated T cells were electroporated with TCR mRNA in an OC-100 processing assembly following 3 T cell-specific protocols: Low, medium or high energy. A) FACS analysis revealed high TCR expression in all 3 transfections. B) 1 x 108 , 2 x 108 and 3 x 108 cells/mL were electroporated with 100 or 200 ug/mL TCR mRNA. FACS analysis demonstrated efficient TCR expression at all 3 cell densities; higher mRNA concentration and cell density led to higher TCR expression.
Scalable Electroporation in Static Processing Assemblies
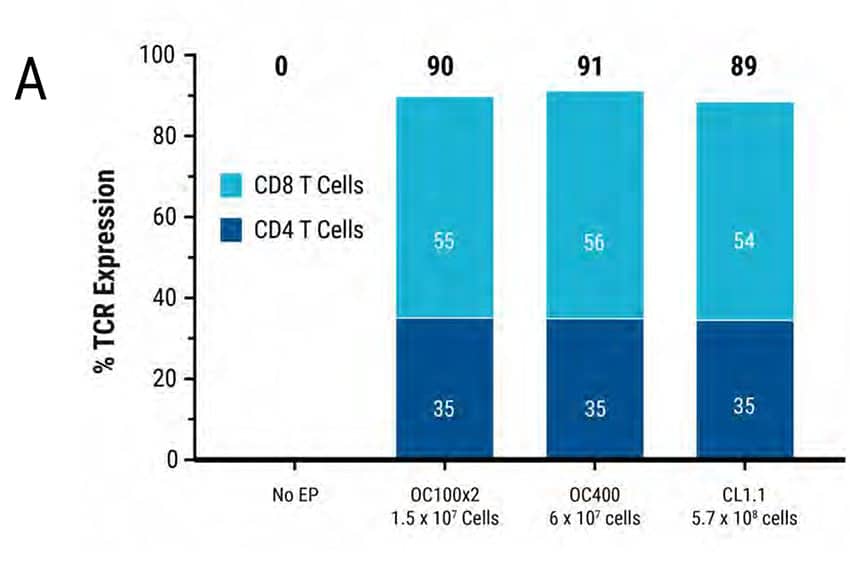

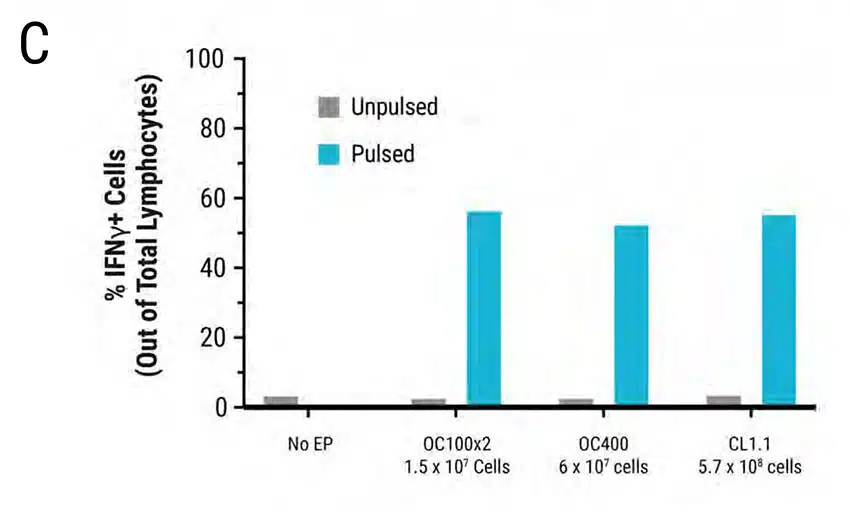
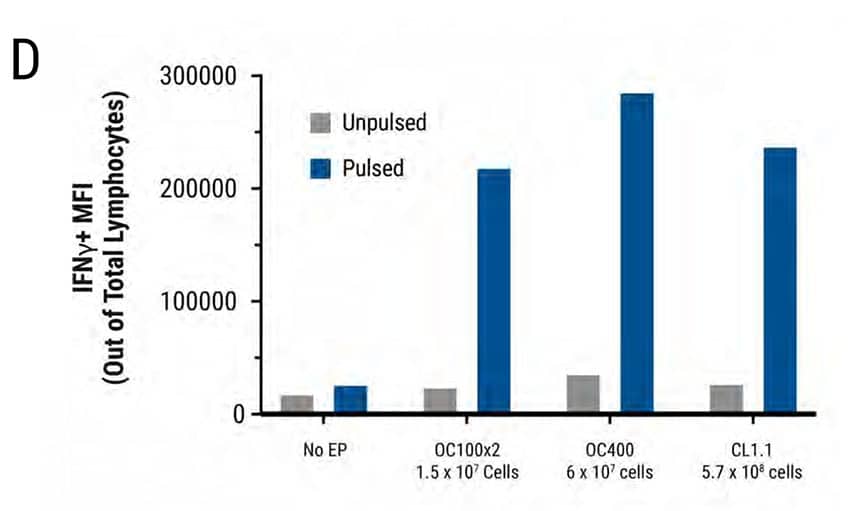
Figure 3: Primary human activated T cells were electroporated in a scalable manner (15 million to 570 million cells) using MaxCyte static processing assemblies, resulting in comparable and high transfection efficiency, cell viability and cytokine function. Primary activated T cells were electroporated with TCR mRNA in MaxCyte static processing assemblies (OC100x2, OC400, and CL1.1 corresponding to a total of 1.5 x 107, 6 x 107, and 5.7 x 108 cells, respectively). A) TCR expression (89-91%) in CD8+ and CD4+ T cell populations, and B) cell viability (81-85%) were similar for cells electroporated in all three static PAs. C-D) The day after electroporation, TCR expressing lymphocytes were co-cultured with antigen peptide-pulsed T2 cells and IFNγ-expressing cells were analyzed by flow cytometry. The percentage of IFNγ-expressing cells and Mean Fluorescent Intensity (MFI) were similar in T cells electroporated with all three static PAs.
Scalable Efficiency in Static and Flow Electroporation Processing Assemblies
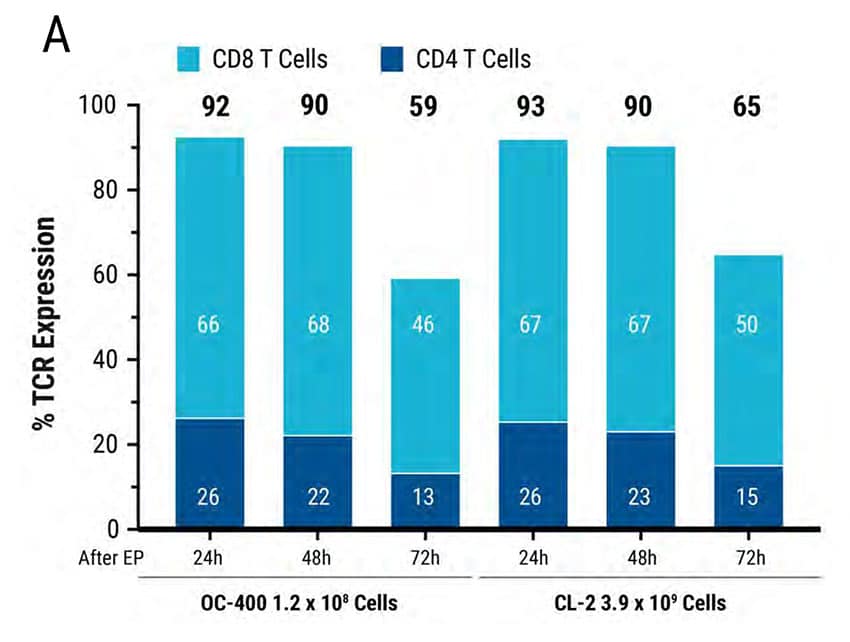

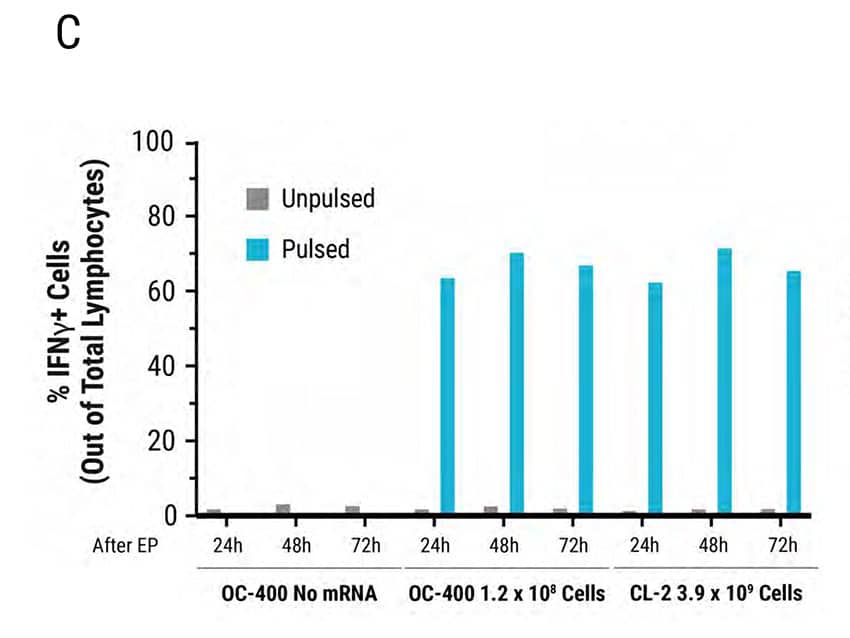
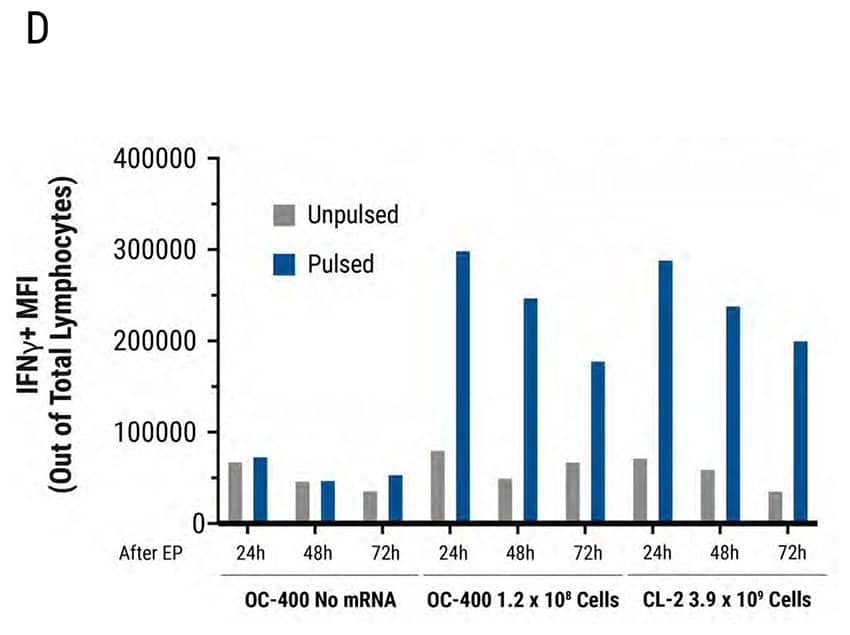
Figure 4: MaxCyte Static and Flow Electroporation results in high electroporation efficiency, viability, and functional cytokine release. Primary activated T cells were electroporated with TCR mRNA in MaxCyte static (OC-400) or flow (CL-2) processing assemblies (PAs). The total number of T cells electroporated in the OC-400 and CL-2 was 1.2E8 and 3.9E9, respectively. T cells were analyzed 24, 48, and 72 hours after transfection by flow cytometry. A) TCR expression was >90% in both PAs for the first 48 hours and dropped to approximately 50% 72 hours after transfection. B) Cell viability was comparable (82-86%) in both PAs over 72 hours. Furthermore, viability did not change in the absence or presence of mRNA. C-D) TCR expressing lymphocytes were co-cultured with antigen pulsed T2 cells and IFNγexpressing cells were analyzed by flow cytometry. C) TCR mRNA electroporated cells had comparable IFNγ expression in both PAs and D) MFI decreased in a time-dependent manner by 72 hours after transfection.
Cryopreservation of Electroporated T Cells
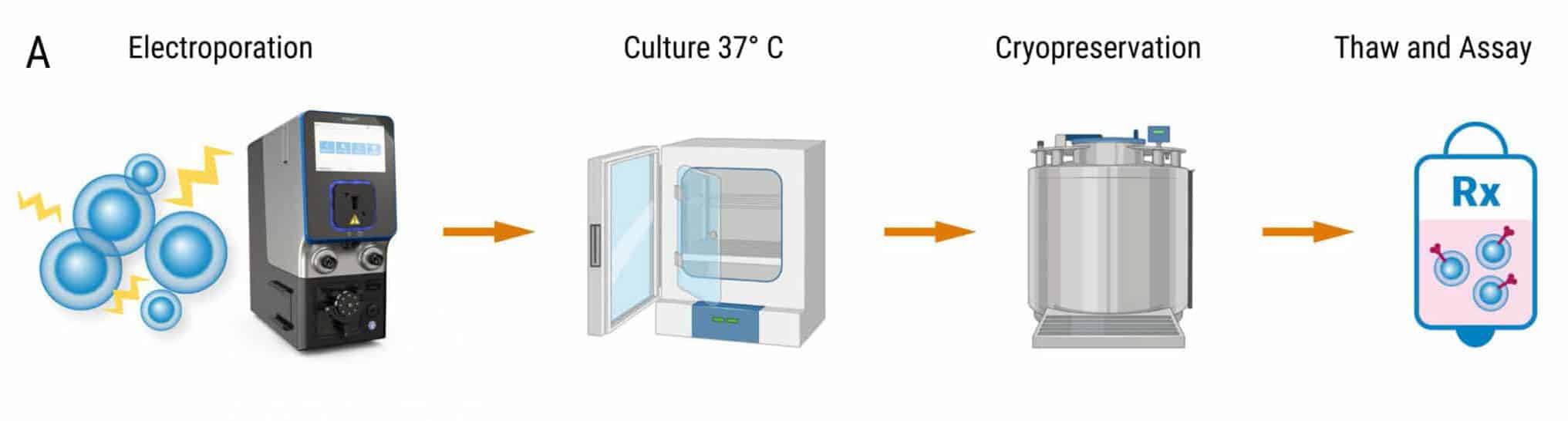


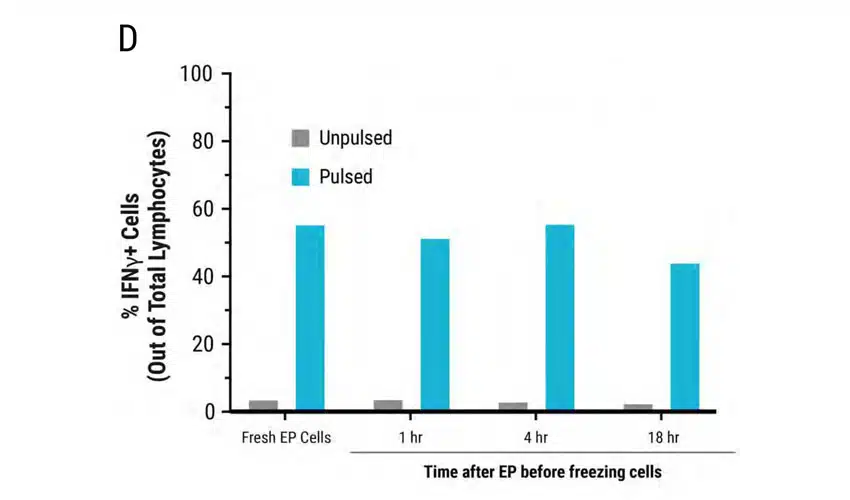
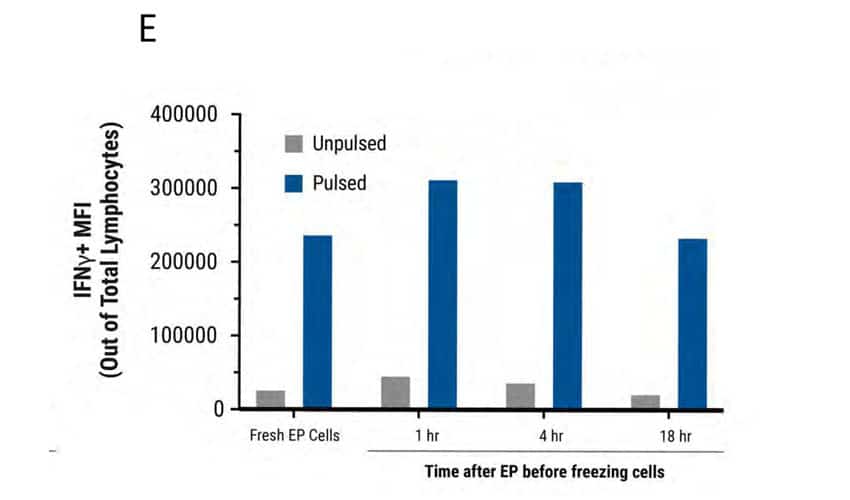
Figure 5: MaxCyte electroporation has minimal impact on TCR expression, cell viability and cytokine function upon cryopreservation. A) Schematic showing primary activated human T cells electroporated with TCR mRNA using a MaxCyte GTx®. Electroporated cells were divided into 4 groups. The first group was cultured for 24 hours at 37°C and analyzed by flow cytometry. The remaining three cell groups were cultured at 37°C for 1, 4, and 18 hours after transfection, and then cryopreserved. After thawing, these three cell groups were cultured for 21, 18, and 4 hours, respectively, and then analyzed at the same time. B) TCR expression was comparable between fresh and frozen electroporated cells (88-89%). C) Cell viability of the frozen cells (75-76%) was not significantly impacted compared with freshly electroporated cells (82%). D-E) TCR expressing lymphocytes were co-cultured with antigen peptide-pulsed T2 cells and analyzed by flow cytometry. IFNγ expression and MFI were comparable between fresh and frozen electroporated T cells.
Summary
MaxCyte Flow Electroporation® Technology provides:
- High transfection efficiency >90% mRNA TCR expression
- High cell viability >80%
- Scalability from 1.5 x 107 cells in static processing assembly to 3.9 x 109 cells in a flow electroporation processing assembly
- Minimal impact on cryopreservation of T cells for high TCR expression, high viability, and functionality comparable to freshly electroporated T cells.





