Flow Electroporation® GMP Processing Assemblies
Flow Electroporation Processing Assemblies for GMP and clinical research applications
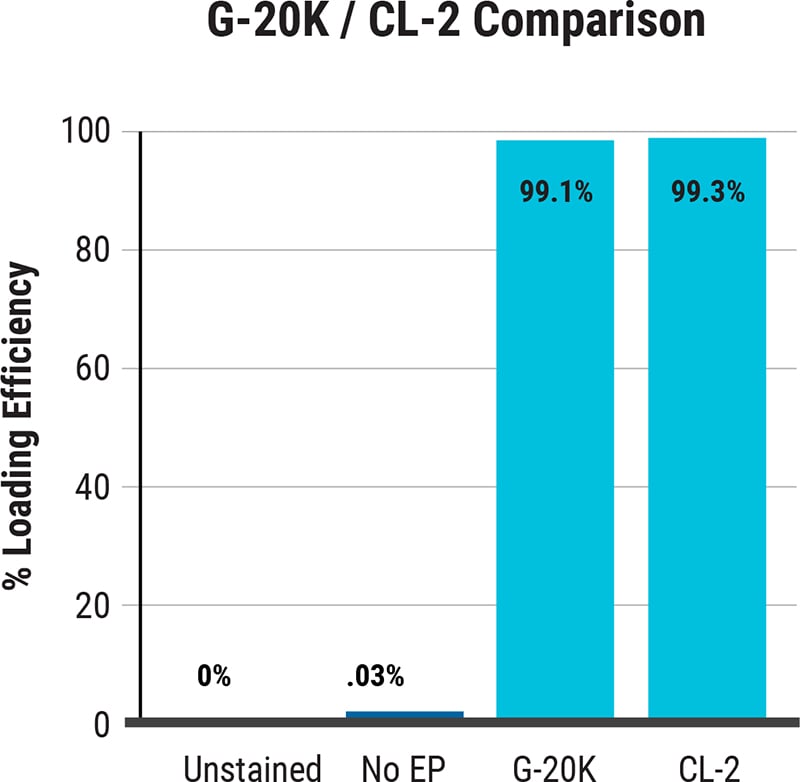
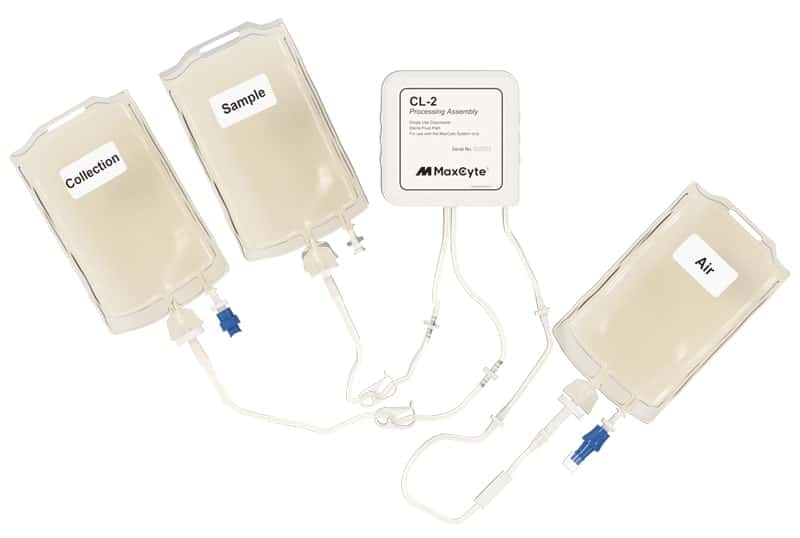
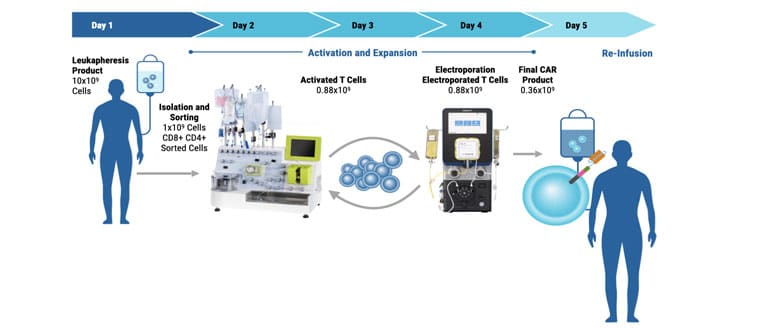
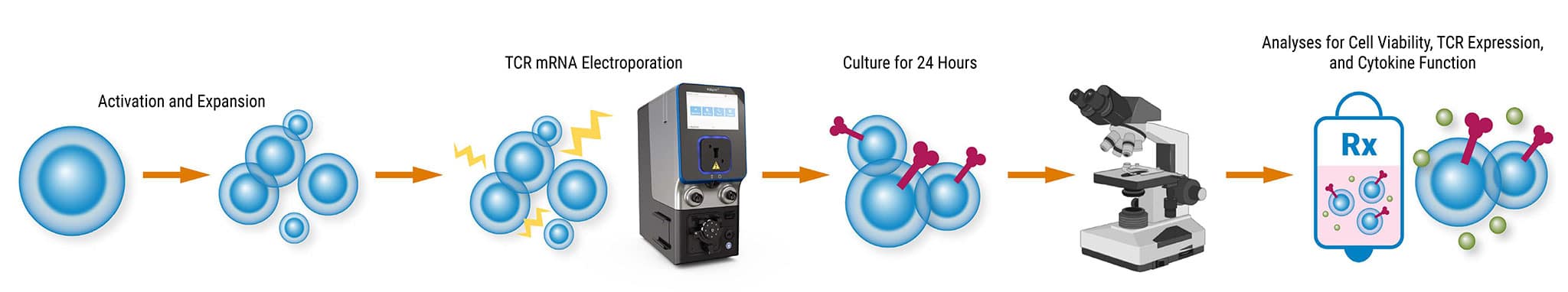
ExPERT™ GMP Processing Assemblies provide the scale and quality standards required for clinical research and production in GMP clean-room facilities. All Processing Assemblies are produced using high quality medical-grade materials that are thoroughly tested for function, sterility, endotoxin, and particulates. These products are included in our master file at the US FDA and similar regulatory agencies globally to support IND applications.
Choose the right processing assembly for your application
Related Reagents and Accessories
Related products to consider using with the Flow Electroporation GMP Processing Assemblies.
Additional Resources
Need additional information or assistance choosing the right processing assembly?