Scientific Brief
Highly Efficient Homozygous Correction of the DYSF Gene in Miyoshi Myopathy Patient iPSCs by ssODN-Mediated Knockin
Abstract
Human induced pluripotent stem cells (iPSCs) play an important role in disease modeling and drug screening, and have tremendous potential for regenerative medicine. Over half all reported pathogenic mutations are caused by single-nucleotide polymorphisms (SNP); as an example, Miyoshi myopathy is a congenital muscle wasting disease caused by a mutation in the dysferlin (DYSF) gene. The correction of such mutations in iPSCs is critical to generating corrected isogenic clones for basic research and for autologous use in clinical applications. However, low efficiencies of correction (<5%) result in time-consuming and laborious screening of many clones to establish an iPSC line. Hotta et al., used the MaxCyte® platform to electroporate 100 bp single stranded oligodeoxynucleotides (ssODN) and CRISPR-Cas9 RNP achieving greater than 70% homozygous SNP correction in the DYSF gene of Miyoshi myopathy patient iPSCs.
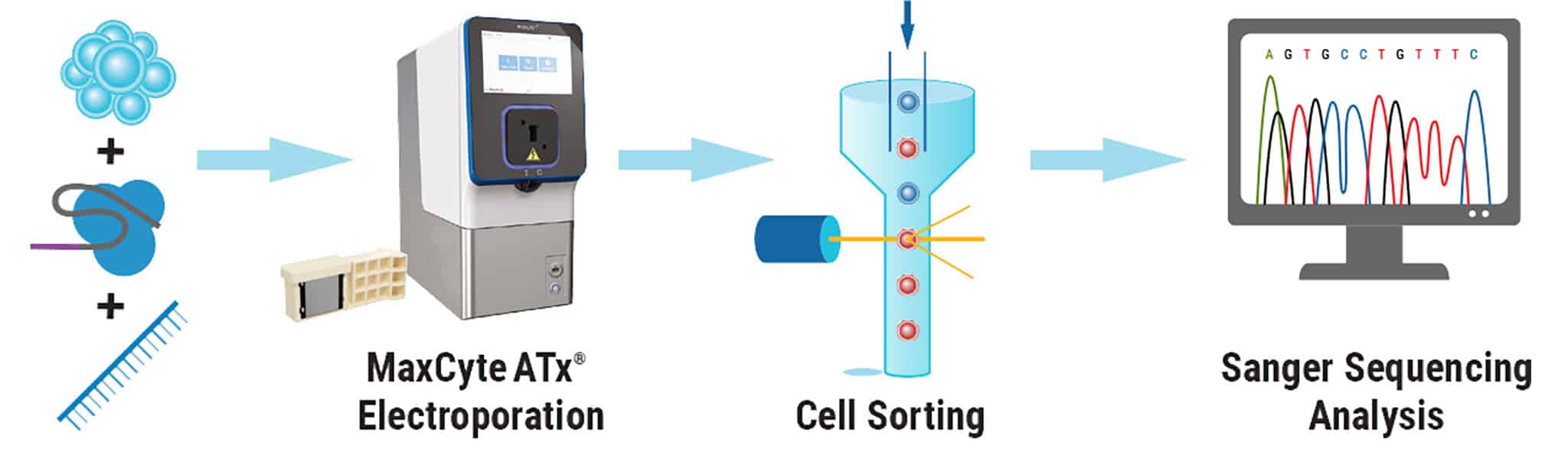
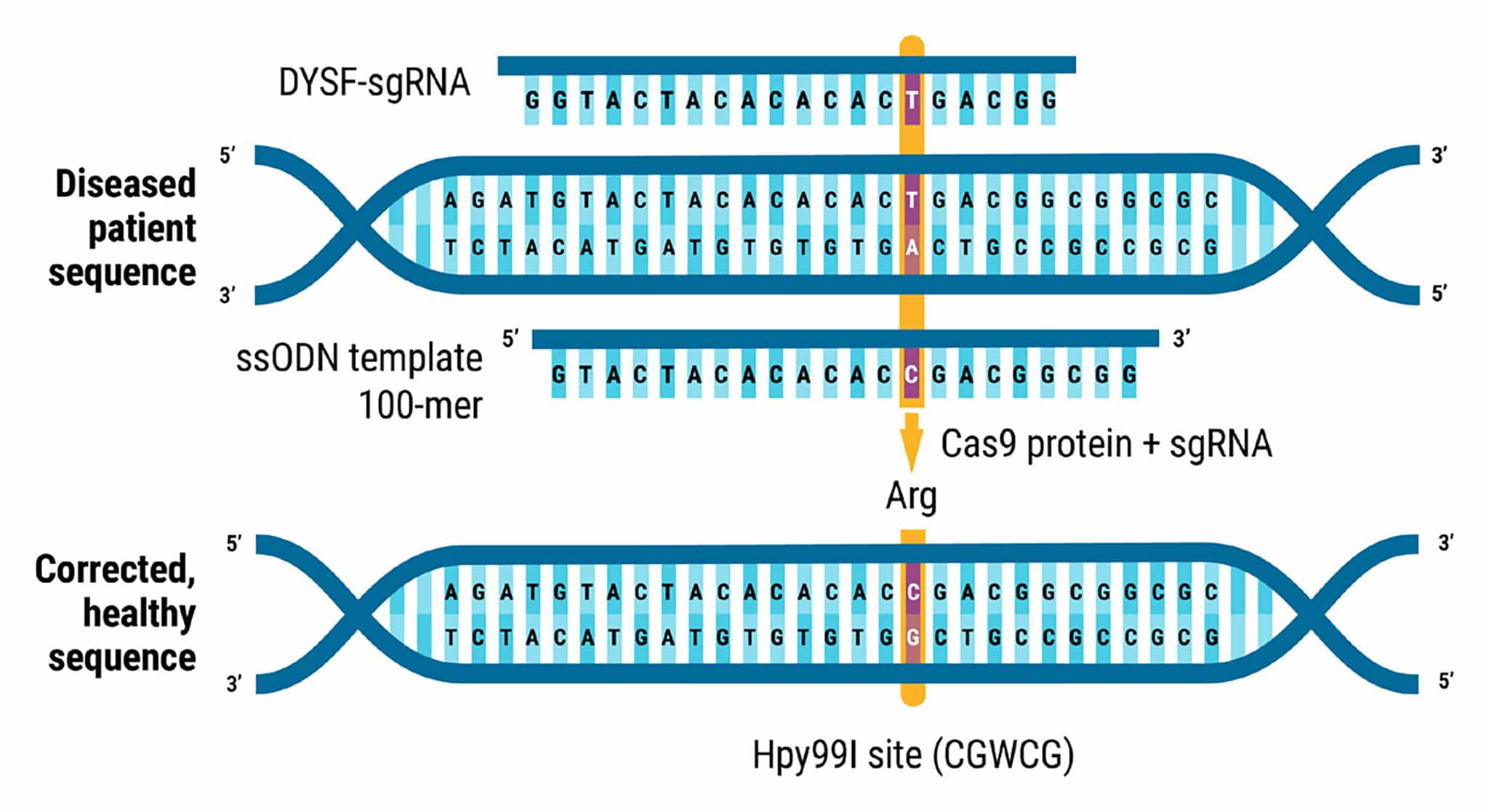
Figure 1. Homozygous Mutation Correction in Miyoshi Myopathy Patient iPSCs. A homozygous nonsense SNP in exon 29 of DYSF in a patient with Miyoshi myopathy (c.C3166T, p.Arg1056Ter, isoform 1, chromosome 2) resulted in the loss of dysferlin protein expression. The mutation was corrected through MaxCyte ATx electroporation (EP) of CRISPR RNP and ssODN repair template using the optimization 8 protocol. Knockin genome editing introduced a new Hpy99I restriction site (CGWCG, W = A or T).
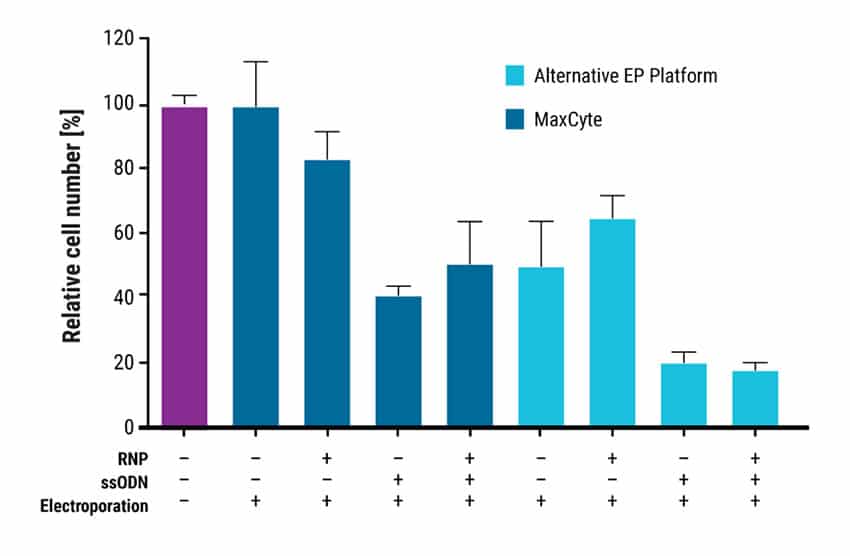
Figure 2. MaxCyte Electroporation Enables Superior Cell Viability. Cell viability was assayed using the Cell Counting Kit-8 (GLPBIO Technology) one day after EP with MaxCyte ATx or an alternative EP platform. Cell viability values relative to a non-transfected control are represented as means ± SD (n = 3, technical triplicate). MaxCyte EP had higher cell recovery than the alternative technology in all conditions tested.
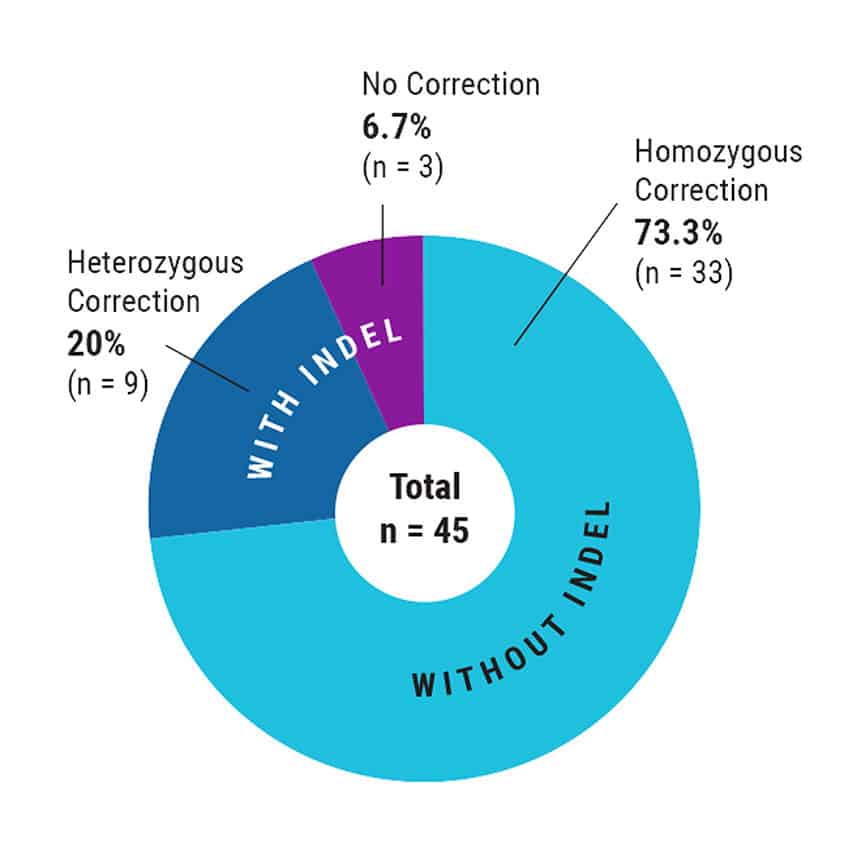
Figure 3. High Efficiency, Homozygous Mutation Correction with MaxCyte Electroporation. After MaxCyte EP of patient-derived iPSCs with ssODN and CRISPR-Cas9 RNP, single cells were plated by fluorescence-activated cell sorting. Subclones were expanded and analyzed by Sanger sequencing to identify over 70% homozygous corrected clones.
Summary
- Footprint-free homozygous correction of a SNP mutation was achieved with over 70% efficiency in the absence of selection.
- MaxCyte electroporation enabled higher cell survival compared to an alternative electroporation platform.
- Higher cell viability post electroporation supports higher HDR frequencies in the final cell population.
- Higher HDR efficiency results in lower cost, less labor and shortened timelines.
- MaxCyte electroporation can be used to transiently transfect a variety cell lines such as iPSCs and stem cells with high transfection efficiencies and cell viability.
Reference
- Kagita A, Lung MSY, Xu H, et al. Efficient ssODN-Mediated Targeting by Avoiding Cellular Inhibitory RNAs through Precomplexed CRISPR-Cas9/sgRNA Ribonucleoprotein. Stem Cell Reports. 2021;16(4):985-996. doi:10.1016/j.stemcr.2021.02.013
This content was adapted from Kagita et al. 2021 under the Creative Commons license Attribution 4.0 International (CC BY 4.0)





