Scientific Poster
In Vitro Characterization of CD19 CAR T Cells Derived by Electroporation of mRNA
Introduction
Cancer immunotherapies reprogram the patient’s immune system to mount a coordinated response against a malignant target. T cells engineered to express Chimeric Antigen Receptors (CARs) represent an effective strategy to target specific cancer cells. Electroporation of mRNA offers several advantages over viral engineering including transient expression of CAR that may limit toxicity, reduce costs associated with viral vector manufacturing and eliminate the risk of virus-associated tumorigenicity. Here, mRNA encoding an anti-CD19 CAR was electroporated into healthy, human T cells using a MaxCyte ATx® instrument.
Experimental Design
CAR T Cell Production using mRNA and Electroporation (EP)
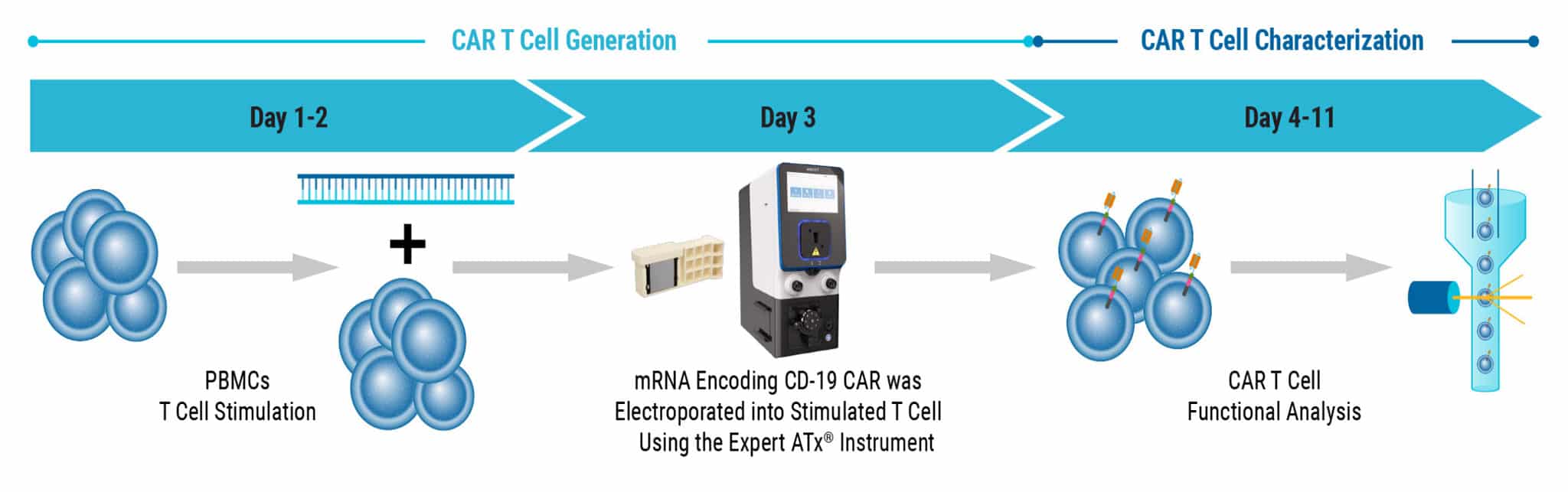
Figure 1
Electroporation of mRNA produces transient transfectants without negatively impacting viability. A) Maxcyte® electroporation (EP) is gentle on cells resulting in high viability. Flow cytometric analysis of Enhanced Green Fluorescent Protein (EGFP) mRNA was used to determine viability and transfection efficiency after EP as follows; control cells (no EP) - 88.7%, mock EP cells (no mRNA) - 87.3%, and cells electroporated with EGFP-mRNA - 88.1%. Highly efficient transfection was achieved with the ExPERT ATx® with 98.6% of the live cells expressing EGFP. B) Each day, EGFP levels were measured using flow cytometry. Daily EGFP analysis indicated mRNA expression was transient. The MaxCyte Expanded T Cell 3 program produced higher levels of EGFP expression than Expanded T Cell 2.
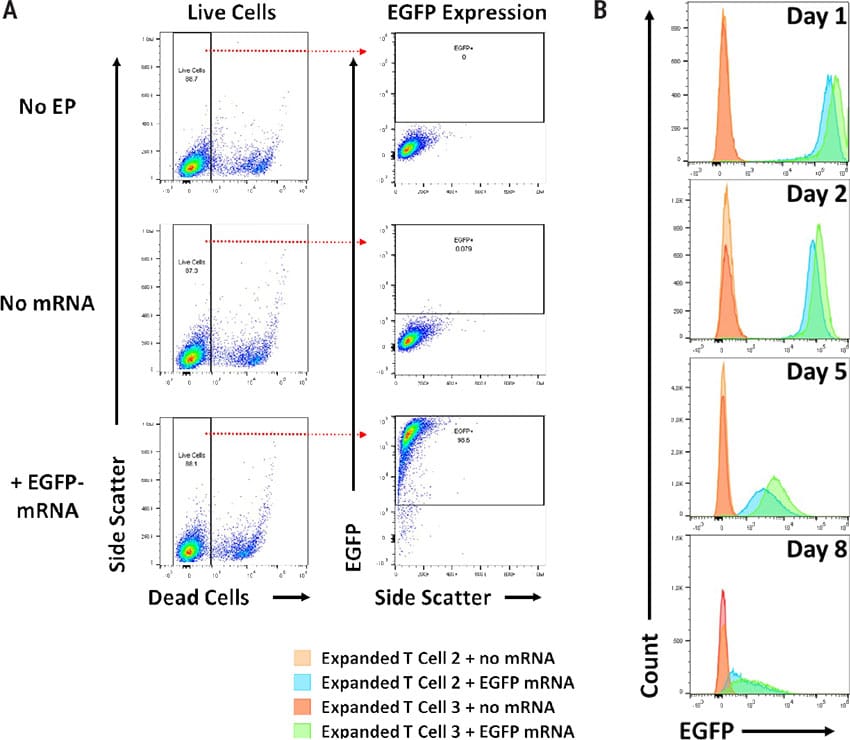
Figure 2

Seamless scale-up of CAR T cell generation. A) Anti-CD19 CAR functional domain organization. B) Diagram depicting flow cytometry staining to identify positive CAR expression. Anti-CD19 CAR mRNA was electroporated into stimulated, peripheral blood mononuclear cells (PBMCs) from healthy donors and flow cytometric analysis was used to determine CAR expression. UTD = untransfected. C) Control CD4+ and CD8+ T cells that did not undergo electroporation and were analyzed for viability and CAR expression. CD4+ and CD8+ T cells were then transfected at both large and small scale. D) Small-scale experiments were performed using the OC-100x2 Processing Assembly where CAR mRNA was electroporated into 5x106 cells. E) The OC-400 Processing Assembly was used for large-scale electroporation of 2x107 cells. High anti-CD19 expression and cell viabilities were observed at both large and small scale.
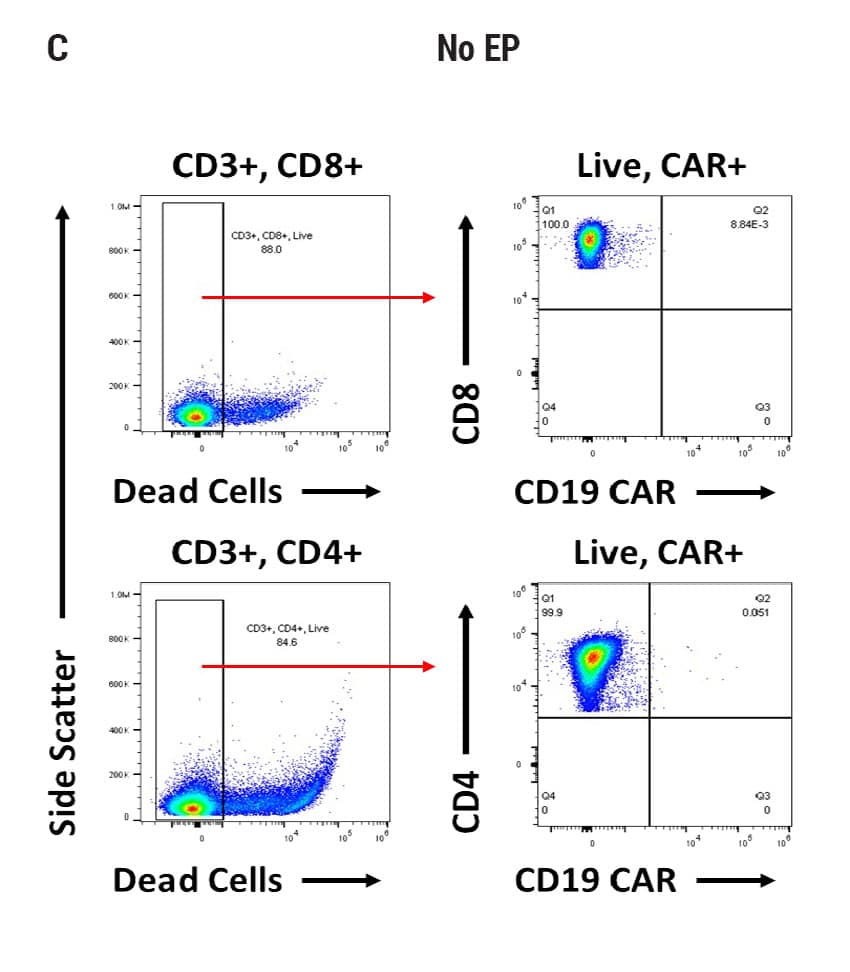
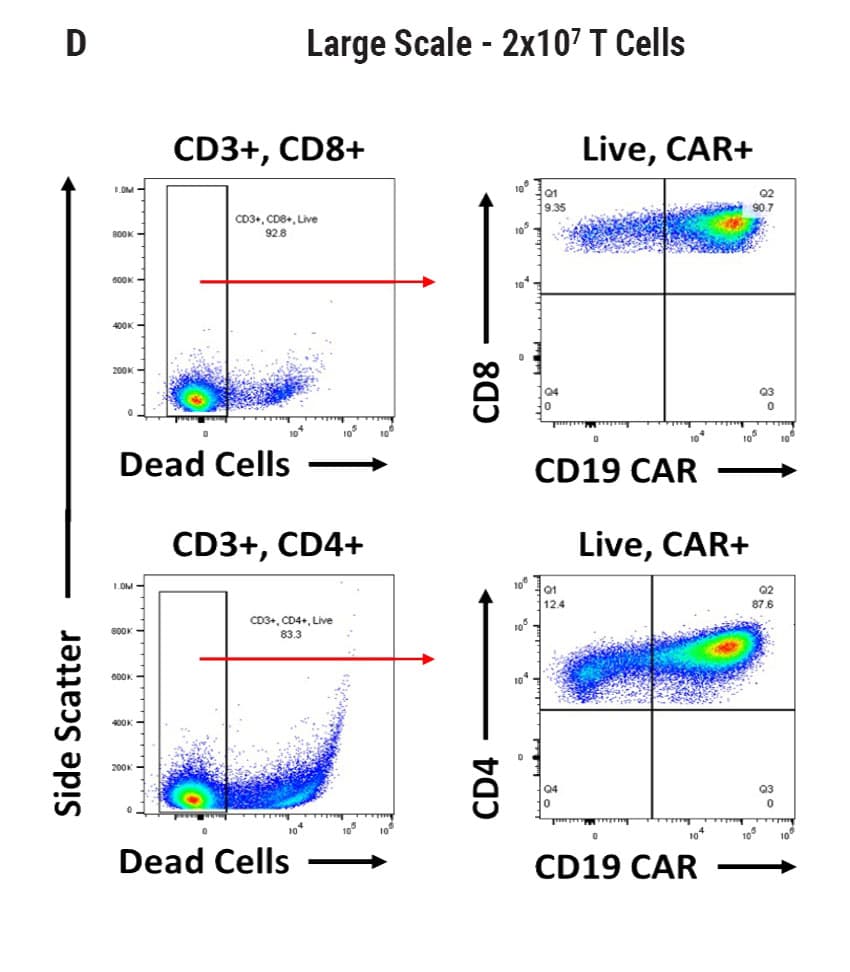
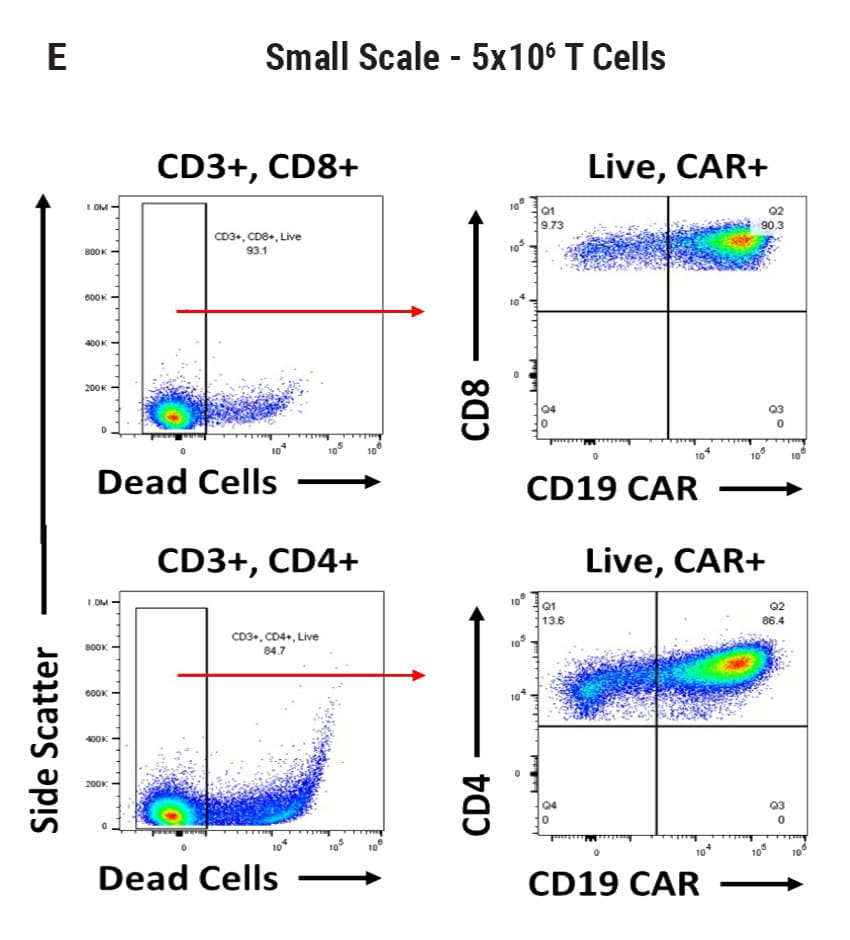
Figure 3
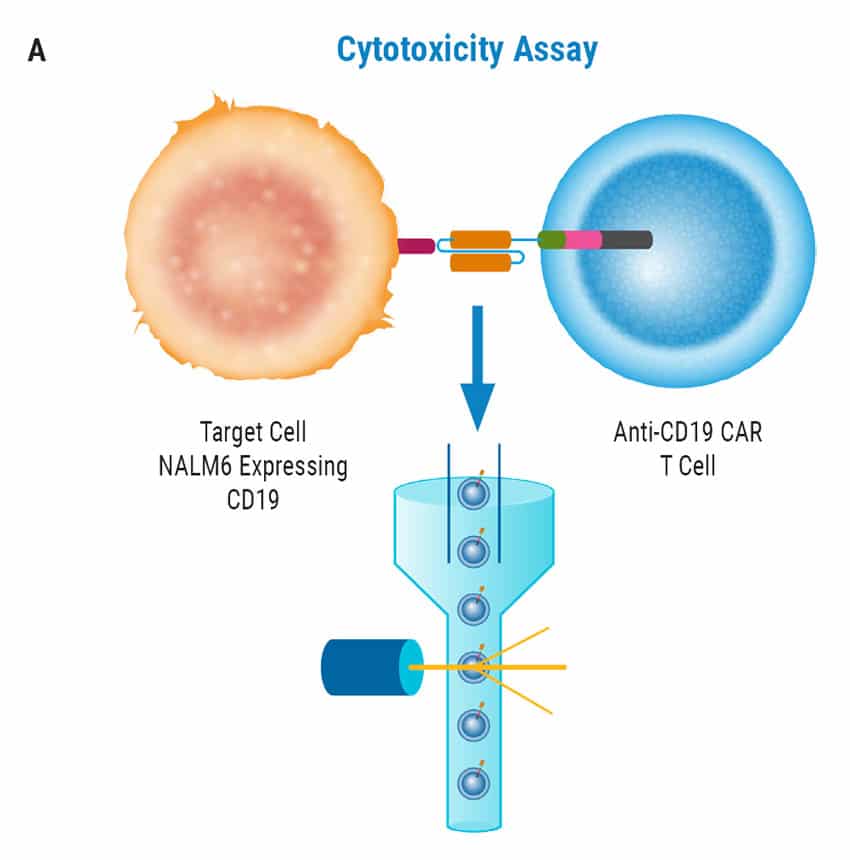
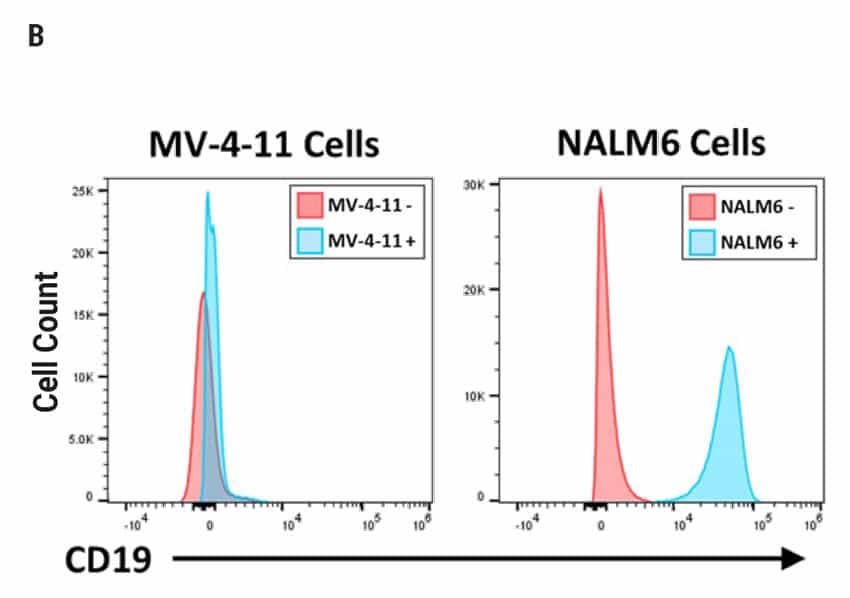
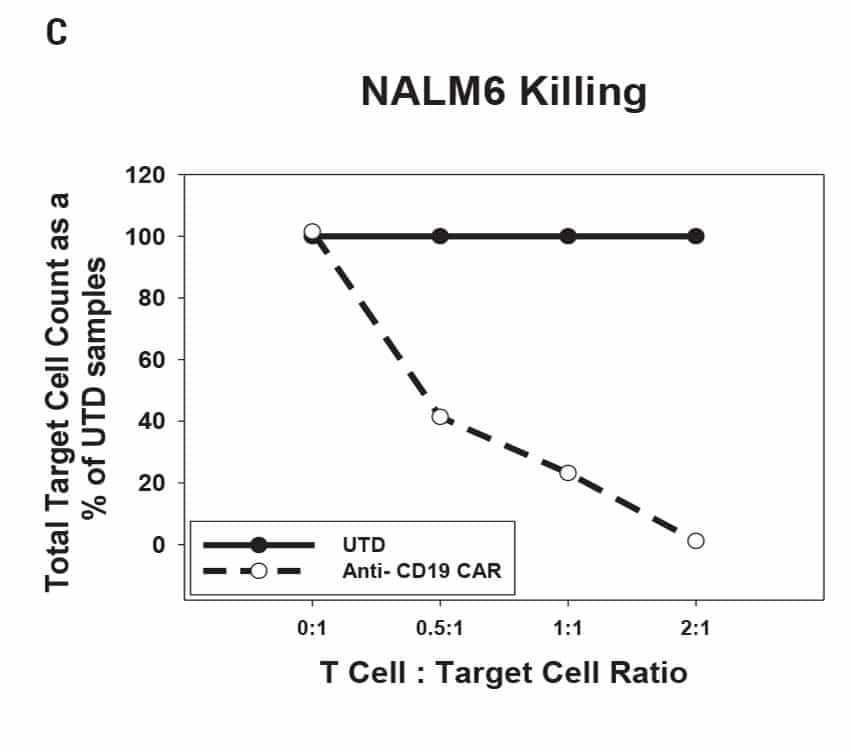
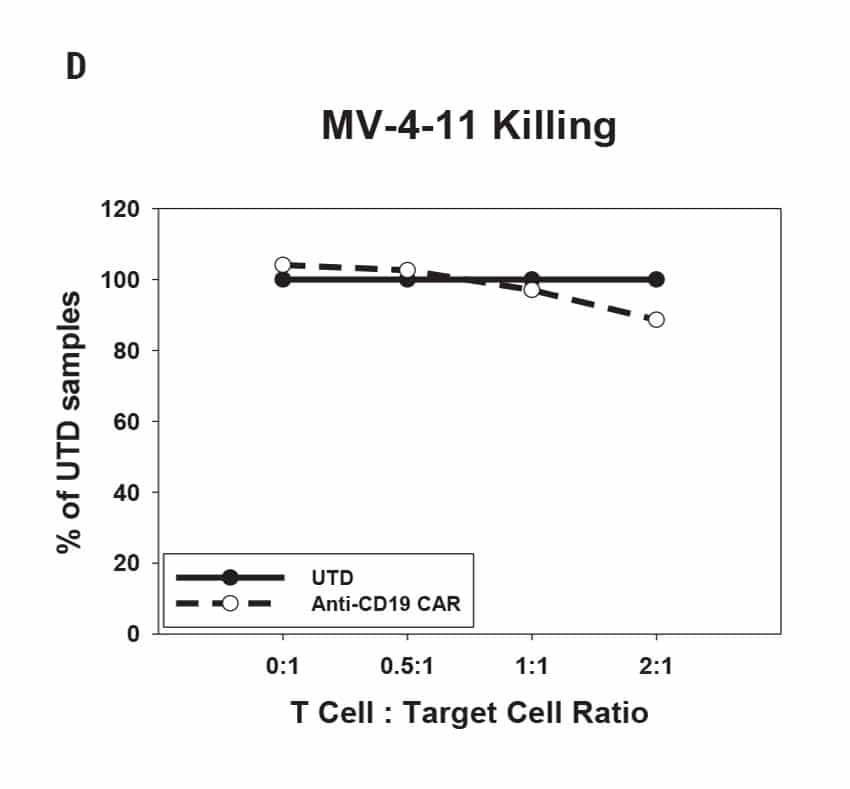
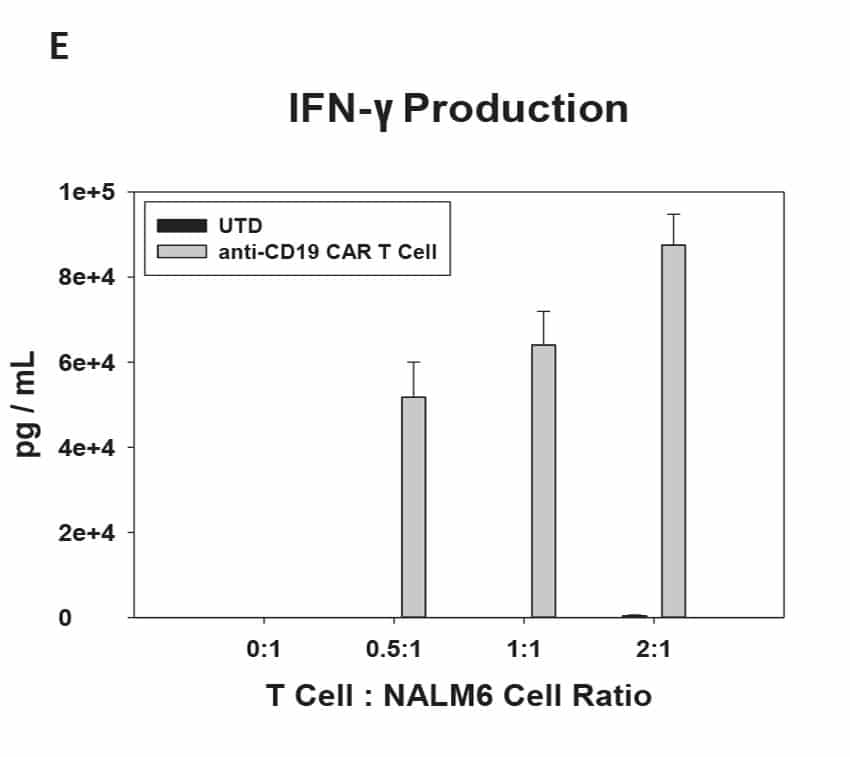
CAR T Cell Functional Characterization A) CAR T cell potency was evaluated via co-culture killing assay, anti-CD19 T cells were cultured with target cancer cells (NALM6 or MV-4-11). Samples were then analyzed by flow cytometry. NALM6, MV-4-11, and T cells were identified with monoclonal antibodies (mAb) specific for their surface proteins and cell survival was identified using viability dye. B) Histograms show MV-4-11 (control) has no CD19 expression in contrast to NALM6 cells. Expression levels were determined in the absence (-) or presence (+) of an anti-CD19 mAb. C) Target and T cell populations from NALM6 cell killing assay were identified by analyzing CD19 and CD3 expression, and total target cells were counted. Engineered CAR T cells are highly potent and effectively killed target cells. 0:1 – 0 T cells for 1 target cell, 2:1 – 2 T cells for 1 target cell. D) Results from MV-4-11 killing assay. CAR T cells are specific. Negligible cell death was observed when engineered T cells were cultured with MV-4-11 cells (no CD-19 expression). Data is representative of triplicate wells for each T cell: Target cell ratio. E) CAR T cell cytokine production was determined using an INF-γ ELISA when T cells were co-culutred with NALM6 target cells.
Summary
- MaxCyte electroporation can be used to transiently transfect a variety of cell lines with high transfection efficiencies and cell viability.
- Development and optimization of electroporation for difficult-to-engineer primary cells is a rapid, straightforward process.
- MaxCyte electroporation provides efficient and scalable delivery of CARs.
- Engineered CAR T cells demonstrated highly potent and specific killing capabilities in vitro.
- Generating functional CAR T cells is the foundation for future research into early drug discovery and new preclinical strategies.





