Application Note
Large-Scale Engineering of Extracellular Nanoparticles for Genome Editing with CRISPR-Cas9 Ribonucleoproteins
Background
Despite advances in human genetics, patients with rare diseases such as Duchenne muscular dystrophy (DMD) still have limited therapeutic options and a short life expectancy. With the advent of CRISPR-Cas9 technology, scientists can now target specific regions of the genome to make precise changes to disease-causing mutations in human cells. In a clinical setting the functional CRISPR components, Cas9 nuclease and single-guide RNA (sgRNA), must be delivered efficiently and directly to the patient’s body. Previous studies in mice have demonstrated that adeno-associated viruses deliver CRISPR-Cas9 effectively, but there is still concern that long-term expression from CRISPR-Cas9 DNA vectors could lead to adverse effects such as non-specific cleavage of important genes involved in cellular function or unintended mutation in oncogenes, which could cause tumors. Moreover, immunological responses to AAV could reduce its efficacy for delivery. For patients to realize the full benefit of recent developments in genomics, clinicians need an alternate delivery system that can transport CRISPR-Cas9 components, transiently, allowing CRISPR-Cas9 to act on a target DNA sequence, make the therapeutic change, and then degrade quickly afterward to limit the chance of adverse events.
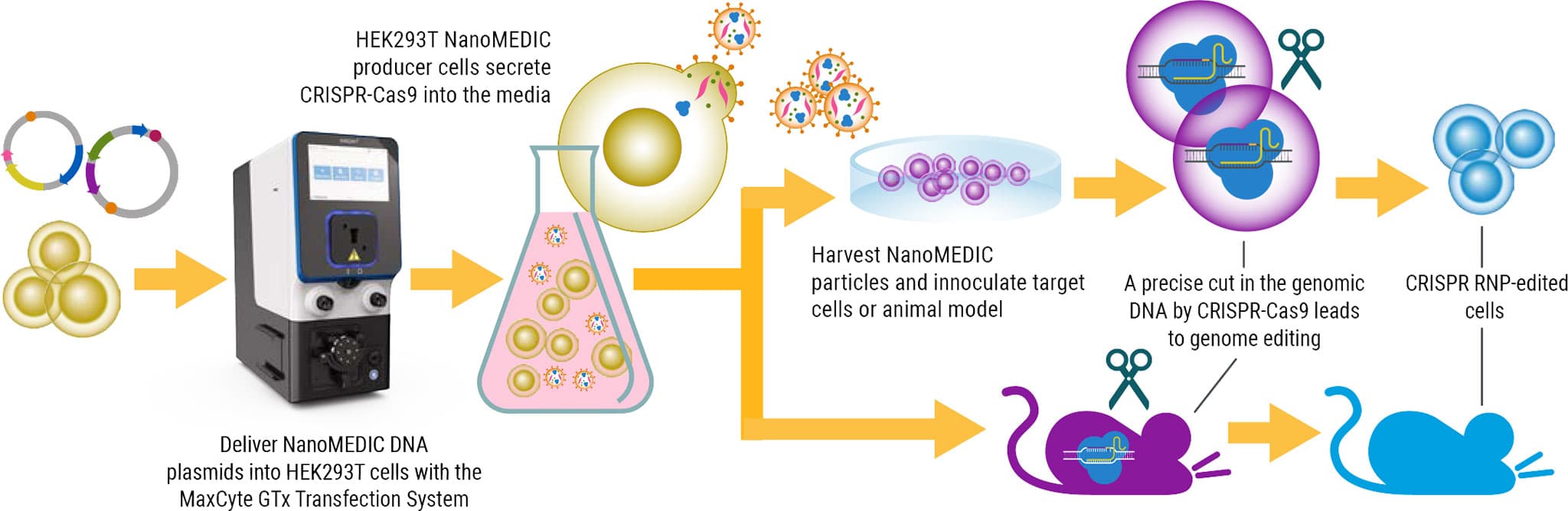
Rationale
Here we feature a recent study published in Nature Communications to develop a new, transient delivery system, termed NanoMEDIC, an engineered extracellular nanovesicle containing a CRISPR-Cas9 ribonucleoprotein (RNP) complex. Following the removal of viral genomic RNA and engineering of structural, lentiviral components, CRISPR-Cas9 RNP was packaged using a chemical ligand-induced dimerization mechanism, retaining the delivery properties of lentivirus without the long-term risks associated with the integration of viral vectors. NanoMEDIC was shown to induce efficient genome editing in a variety of cell types including muscle myoblasts, T cells, human iPSCs, and monocytes as well as in vivo in immunodeficient mice. However, while the production of NanoMEDIC was feasible at small-scale for basic research, the researchers sought to develop a scalable method to produce NanoMEDIC using a cGMP compatible, Flow Electroporation® system which could be applicable for industrial manufacturing.
Technical Approach
To adapt the production process to clinical standards, HEK293T suspension culture was used, eliminating animal components and improving cell handling through higher density cell culture. Next, electroporation conditions were optimized to increase NanoMEDIC production. NanoMEDIC yield was enhanced by reducing the amount of VSV-G envelope plasmid in the transfection mixture, and DNase treatment of transfected production cells, post electroporation, increased cell viability. A customized, high-electroporation-energy protocol increased NanoMEDIC production. Importantly, while conditions for electroporation were established at a small scale using 60 x 106 cells, scalable MaxCyte Flow Electroporation® allowed for scale-up to production of NanoMEDIC cells at 1.2 x 109 using flow electroporation. Yields between small-scale and large-scale NanoMEDIC production were confirmed to be comparable by quantification of active CRISPR-Cas9 RNP complex in an in vitro enzymatic assay.
Conclusion
This study establishes a transient, CRISPR-Cas9-delivery system that holds potential not only for gene therapy but also for delivering other cargo, such as viral antigens, that could be applied to vaccine development to elicit a therapeutic immune response. The manufacturing process developed here is animal component-free and utilizes MaxCyte Flow Electroporation® for scalable NanoMEDIC production of up to billions of cells.
Reference
Gee P, Lung MSY, Okuzaki Y, et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat Commun. 2020;11(1):1334. Published 2020 Mar 13. doi:10.1038/s41467-020-14957-y





