Scientific Brief
Multi-gene Knockdown for Improved Therapeutic Efficacy
Abstract: Non-viral Engineering Approaches for Creating TCR Knockout Cells and Expressing Synthetic T Cell Receptors
Manufacturing of CAR T cells from autologous patient cells continues to face a multitude of challenges which ultimately can limit the treatable patient population and lead to unpredictable and highly variable clinical outcomes. Researchers are looking to sophisticated gene editing to engineer potent CAR T cells that are resistant to the immunosuppressive tumor microenvironment, ultimately using a universal cell source derived from healthy human cells. In this poster we present data for the non-viral delivery of mRNA and CRISPR-Cas9 machinery using MaxCyte® Flow Electroporation® Technology for knockout of endogenous TCRs and expression of engineered therapeutic CAR/TCRs. We demonstrate high efficiency delivery with low primary cell toxicity at clinical-scale — all critical parameters for commercial production of quality, clinically-active genetically engineered cells.
Case Study
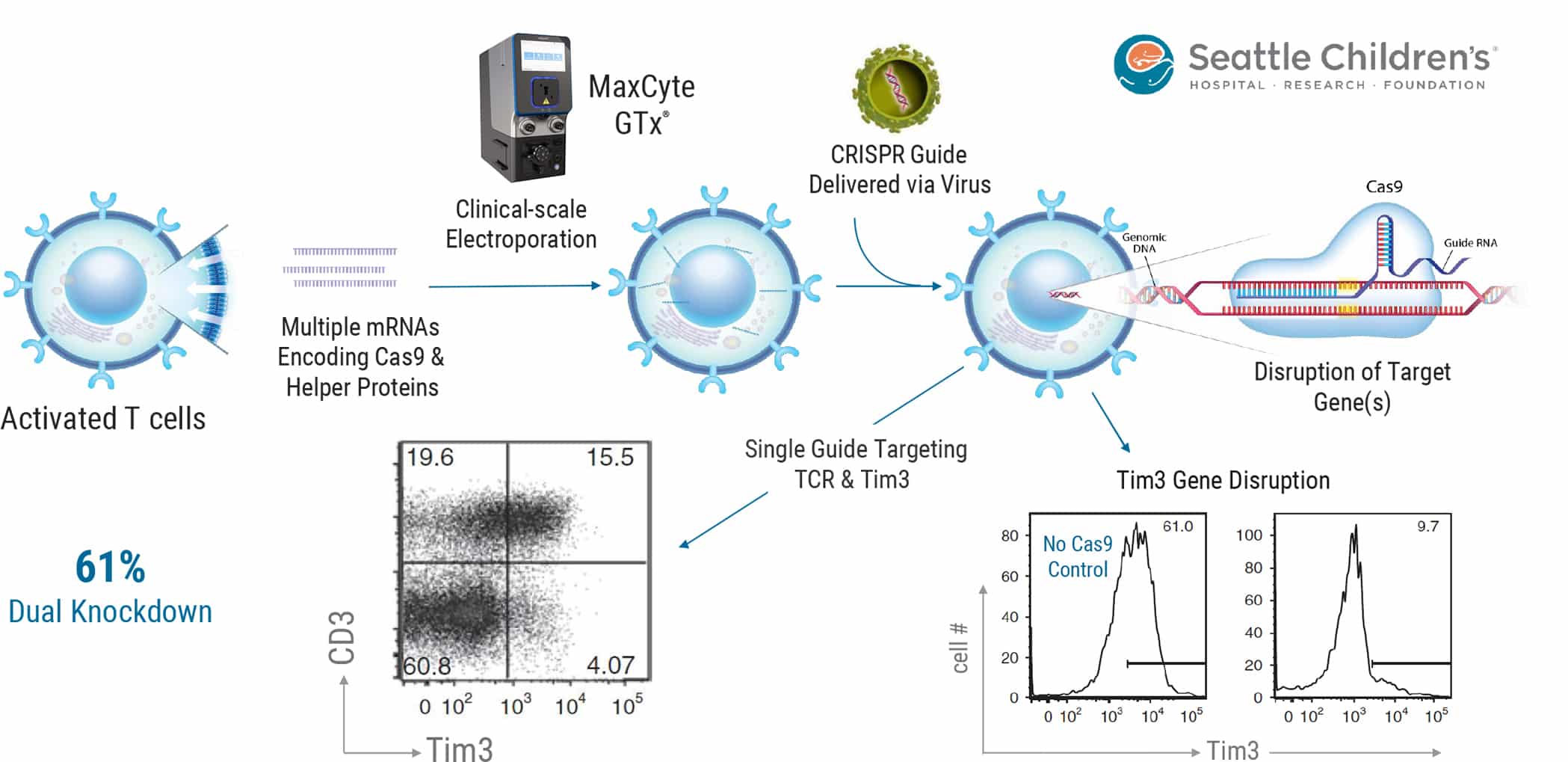
Gene editing is being used to improve cell-based therapies, including disruption of checkpoint protein genes to create engineered cells refractory to tumor immunosuppression or knockdown of endogenous TCRs to reduce mispairing of endogenous and engineered TCRs and/or to create allogeneic donor cells. The work published in Mol Ther., 24(9), 1570- 1580, 2016 and summarized above augmented their previous virus-based CRISPR-mediated delivery method with the MaxCyte GTx®. Activated T cells were first electroporated with multiple mRNAs encoding Cas9 and AAV helper proteins. Electroporated cells were then transduced with AAV for delivery of CRISPR gRNA targeting Tim3 alone or Tim3 & CD3. The dual gRNA targeting of both Tim3 and CD3 led to >60% knockdown of both proteins as assessed via FACS. See publication for detailed methods.
Summary
- Non-viral engineering enables rapid development of next-generation therapies — such as endogenous T cell receptor knockout or disruption of checkpoint inhibitors — with the benefit of simplified, more cost-effective manufacturing.
- MaxCyte Flow Electroporation® Technology (co)delivers a diversity of payloads including mRNA, sgRNA, RNPs, and plasmid & minicircle DNA providing flexibility for sophisticated, non-viral engineering including:
- Transient mRNA expression
- Nuclease-mediated gene editing (CRISPR, TALEN, ZFN)
- Transposon insertion (Sleeping Beauty®, piggyBac®)
- MaxCyte clinical scalability and regulatory compliance provide for streamlined clinical translation of new therapies.
- The high efficiency and low toxicity of MaxCyte Flow Electroporation provides for strong expression of exogeneous genes such as CARs and/or high gene disruption frequencies that bolster therapeutic efficacy.
- Development and optimization of MaxCyte Flow Electroporation for new cell types and payloads is a rapid, straightforward process.





