Application Note
MaxCyte® Flow Electroporation Enables Immunocytokine Development
Background
Immunotherapy is a cutting-edge approach to cancer treatment whereby a patient’s immune system is directed to target and kill tumor cells. T cell activation and expansion are essential for a successful anti-tumor response, and cytokines such as IL-2 have been investigated as cancer therapeutics. Unfortunately, systemic administration of IL-2 (similarly to many other cytokines) causes significant toxicity.
An alternative strategy uses antibodies against cancer-specific markers to trigger an immune response. While this approach has been widely successful in lymphoma, solid tumors have proven harder to treat. Despite precise antigen binding, anti-tumor antibodies often fail pre-clinically due to a lack of direct anti-tumor activity. A novel class of fusion proteins has been developed to overcome the limitations of these early immunotherapeutic approaches. Immunocytokines (ICK) combine antibody specificity with the therapeutic efficacy of cytokines.
Many solid tumors overexpress carcinoembryonic antigen (CEA)-related cell adhesion molecule 5, making it an excellent tumor marker. An anti- CEA-IL-2 immunocytokine could be an effective therapeutic strategy against CEA-expressing tumors. To generate anti-CEA-IL-2 proteins, plasmids encoding the ICK light and heavy chain fusion must be delivered into CHO cells in a specific ratio. The MaxCyte STX™ delivered multiple vectors with high efficiency and low toxicity enabling the expression of correctly assembled, functional anti-CEA-IL-2 protein. MaxCyte Flow Electroporation® technology streamlined ICK manufacturing and is helping accelerate it into the clinic.
MaxCyte Enabled Workflow for Protein Expression
Aim
In this innovative study, a novel anti-CEA-IL-2 ICK was developed by combining a full-length human IL-2 with M5A, a clinically-tested, fully-humanized anti-CEACAM5 antibody. MaxCyte’s Flow Electroporation technology generated fully bioactive ICK in amounts sufficient for pre-clinical studies. The anti-CEA-IL2 ICK was tested for anti-tumor activity in murine breast and colon cancer models.
Method Overview
Outlined below is a rapid and efficient protocol for the manufacture and purification of the anti-CEA-IL-2 ICK.
Plasmid Generation: A human IL-2 gene was fused to the 3’-end of the M5A heavy chain gene. The construct was amplified by PCR and sub-cloned into the pcDNA3.3-TOPO-TA expression vector.
Transfection: CHO-S cells were mixed with plasmids encoding the M5A heavy chain-IL-2 fusion and M5A light chain in a 3:1 molar ratio. Plasmids encoding the M5A heavy and light chains were similarly mixed and used as a control. Large-scale transient transfection was performed using MaxCyte STX Flow Electroporation. Cells were then transferred to a 20 L WAVE™ bioreactor system and grown in serum-free media at 37° C for one day.
Expansion: A feed strategy optimized by MaxCyte to enhance viability and protein production was used. Cells were fed daily with a nutrient-rich supplement and incubated at 32° C for 14 days.
Protein Harvest: Supernatant containing anti-CEA-IL2 was collected and purified using ion-exchange chromatography. Protein was quantified and characterized before use in immunotherapy studies in mice.
Results
ICK Fusion Protein Expression
MaxCyte STX electroporation delivered anti-CEA-IL2 ICK expression plasmids to CHO cells. Transfected cells produced 0.12 g/L of ICK (Fig. 1) and a total of 0.435 g of the fusion protein was purified from a 20 L bioreactor. MaxCyte electroporation is gentle on the cells, ensuring high cell viability during the 14- day expansion period (Fig. 1).
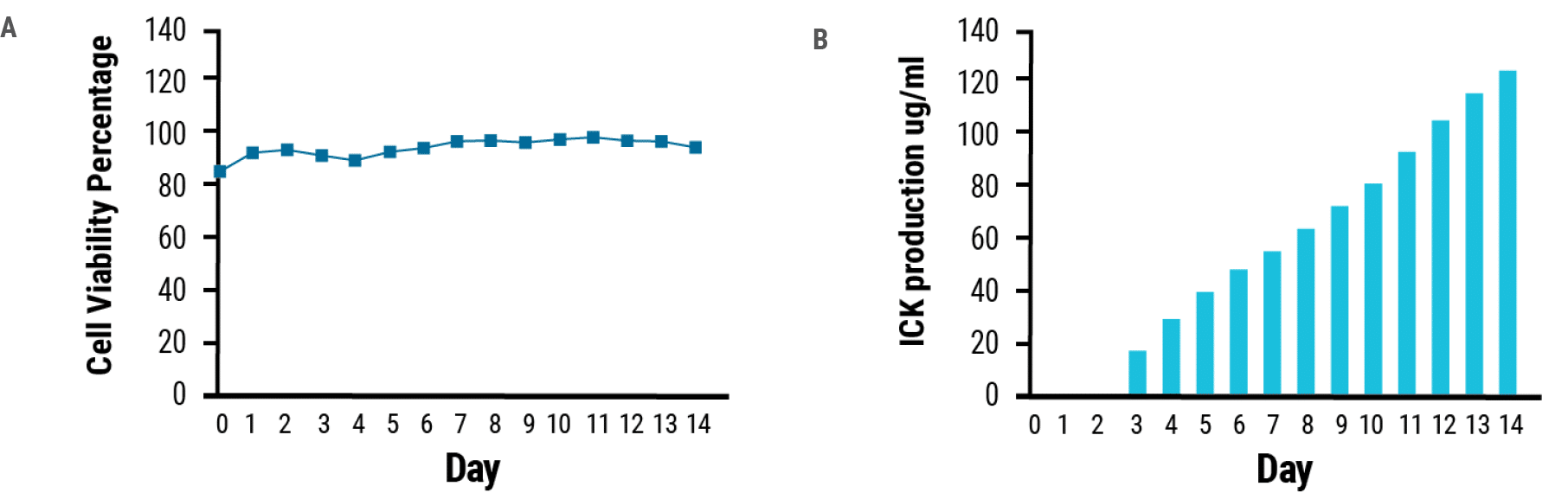
Figure 1. ICK production. A) viability and B) ICK production are plotted over the course of 14 days post electroporation.
ICK Fusion Protein is Biologically Active
Following electroporation, the M5A light chain and heavy chain-IL2 fusion must assemble correctly to form the functional immunocytokine. Purified anti-CEA-IL-2 was analyzed by SDS gel electrophoresis. The high molecular weight bands obtained under non-reducing conditions (Fig. 2A) produced distinct heavy and light chain bands under reducing conditions (Fig. 2B). The immune activity of the ICK was similar to that of IL-2 (Fig. 2C).
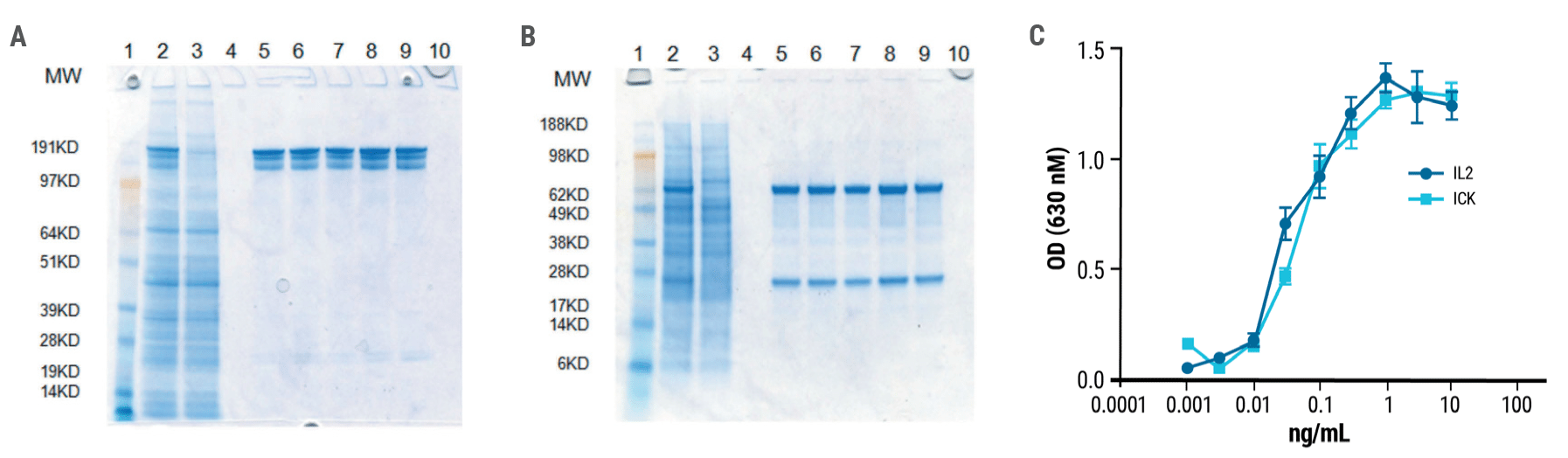
Figure 2. Anti-CEA-IL2 ICK is a fully functional fusion protein. Gel electrophoresis of purified ICK was performed under A) non-reducing and B) reducing conditions. Single bands were obtained for both heavy and light chains under reducing conditions. C) ICK activity was measured using a HEK-BlueTM IL-2 reporter cell line. The activity of the anti-CEA-IL-2 ICK was comparable to that of recombinant human IL-2.
ICK Suppresses Tumor Growth
Murine models of CEA+ breast and colon cancer were generated by injecting tumor cells into CEA transgenic mice. Once the mice had tumors, they were given five daily injections of ICK (therapeutic) or M5A antibody (control). Compared to control, ICK-treated mice developed significantly smaller tumors over time (Fig. 3A). As radiation treatment is a standard therapeutic option for patients with cancer, researchers wanted to determine whether the combination of radiation and ICK would improve therapeutic outcomes. Mice with tumors were irradiated with stereotactic radiation (SRT) followed by four daily injections of either ICK or M5A antibody. Combination therapy reduced tumor growth to a greater extent than control treatment in both breast and colon cancer models. (Fig. 3B, 3C). Using multiple lower doses of radiation instead of a single high dose may boost the effectiveness of immune therapy. Therefore, mice were administered either a single dose of high-intensity 10 Gray (Gy) SRT or four daily fractions of 2.5 Gy SRT, both followed by four doses of ICK. Fractionated SRT combination therapy was more effective than single-dose SRT combination therapy, resulting in tumor eradication in half the mice treated (Fig 3D).
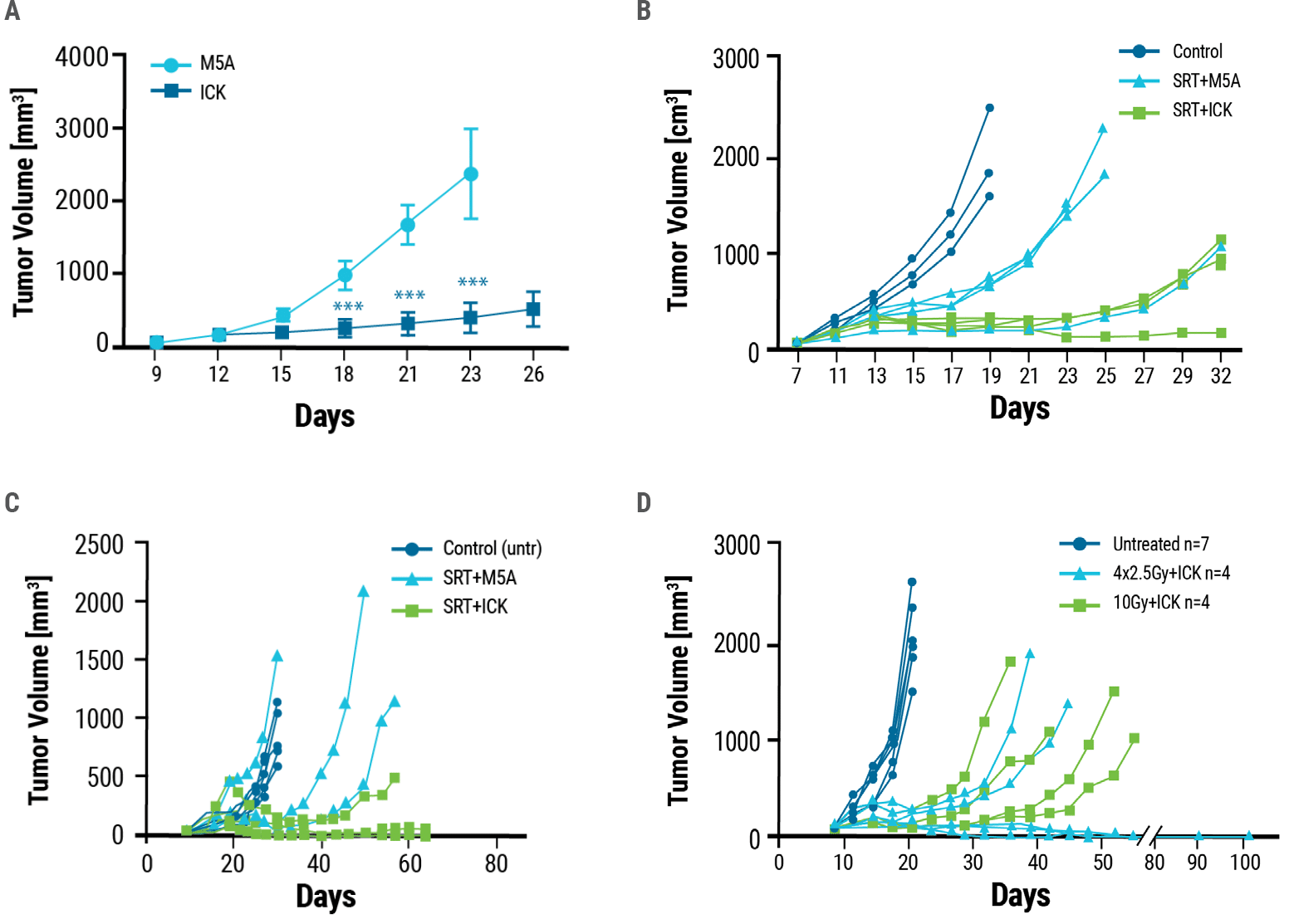
Figure 3. Anti-tumor effects of ICK treatment. A) Tumor-bearing CEA transgenic mice were dosed with five daily injections of 25 μg of ICK or M5A. ICK inhibited tumor growth significantly, whereas M5A mice were euthanized by day 26 due to excessive tumor growth. Mouse models of B) breast and C) colon cancer were exposed to a single dose of 10 Gy stereotactic radiation alone (SRT+M5A) or stereotactic radiation followed by four doses of ICK (SRT+ICK). D) ICK in combination with fractionated SRT, 4 doses of 2.5 Gy, reduces tumor growth and eradicates tumors in half of the treated mice. One experiment of two is shown as a representation, n = 4–7 per group.
Conclusion and Future Applications
In summary, MaxCyte is helping advance ICK therapeutics into the clinic by enabling simple, reliable and consistent production of fusion proteins. MaxCyte Flow Electroporation technology can generate pre-clinical amounts of bioactive and immunoactive anti-CEA-IL-2 ICK that demonstrated anti-tumor efficacy in breast and colon cancer murine models. The study also showed the benefits of using ICKs with other therapeutic approaches such as stereotactic radiation. Prolonged survival and development of anti-tumor immunity following combination therapy showcases the promise of anti-CEA-IL-2 ICK in clinical trials.
ICKs provide antibody-mediated targeted delivery of cytokines to tumors combining specificity with therapeutic efficacy. Several IL-2, IL-12 and TNF-based ICKs are in various phases of preclinical and clinical development.2,3 MaxCyte’s cGMP-compliant, scalable technology enables rapid, high-yield protein expression that can compress timelines and accelerate immunotherapies from concept to clinic.
References
- Kujawski M, Sherman M, Hui S, et al. Potent immunomodulatory effects of an anti- CEA-IL-2 immunocytokine on tumor therapy and effects of stereotactic radiation. Oncoimmunology. 2020;9(1):1724052. Published 2020 Feb 14. doi:10.1080/21624 02X.2020.1724052
- Runbeck E, Crescioli S, Karagiannis SN, Papa S. Utilizing Immunocytokines for Cancer Therapy. Antibodies (Basel). 2021;10(1):10. Published 2021 Mar 9. doi:10.3390/antib10010010
- List T, Neri D. Immunocytokines: a review of molecules in clinical development for cancer therapy. Clin Pharmacol. 2013;5(Suppl 1):29-45. Published 2013 Aug 20. doi:10.2147/CPAA.S49231
Reprinted by permission of the publisher Informa UK Limited trading as Taylor & Francis Ltd, http://www.tandfonline.com
* Since publication of this study, the new ExPERT STx® instrument has been released to include enhanced software, an improved user interface and integrated touch screen.





