Scientific Poster
Scalable Electroporation of Adult Keratinocytes with Multiplexed CRISPR Ribonucleoproteins for the Development of Novel Cell Therapeutics
Abstract
The use of engineered keratinocytes for cell therapy has long been an attractive option to treat various dermatological, oral, and aural disorders. Keratinocytes are easily adapted to in vitro culture and expanded from even small tissue specimens, making them an ideal cell type for a range of autologous and allogeneic cell therapy products. However, engineered keratinocyte-based cell therapies have been limited by the lack of efficient transfection methods for adult keratinocytes. Here we aimed to develop a GMP-compliant, scalable cell engineering process using MaxCyte® electroporation to transfect neonatal and adult primary keratinocytes from four distinct anatomical locations.
Using MaxCyte, we efficiently delivered mRNA or CRISPR-Cas9 ribonucleoproteins (RNPs) to primary keratinocytes from the foreskin, arm, tonsils, and tympanic membrane at two different scales. This was accomplished without compromising cell viability, morphology, or growth capability. Furthermore, delivery of multiple CRISPR RNPs in a single electroporation achieved highly efficient, multiplexed gene editing in a simple, adaptable process. These improvements in transfection efficiency and cell viability reduced keratinocyte engineering times by up to 4 weeks, with a significantly higher success rate than a standard chemical transfection method. Finally, we demonstrate the scalability of the MaxCyte electroporation process, enabling the engineering of millions of primary keratinocytes without any loss of efficacy. In summary, the MaxCyte ExPERTTM platform provides efficient, GMP-compliant, multiplexed transfection for the development and scalable production of engineered keratinocyte cell therapy products.
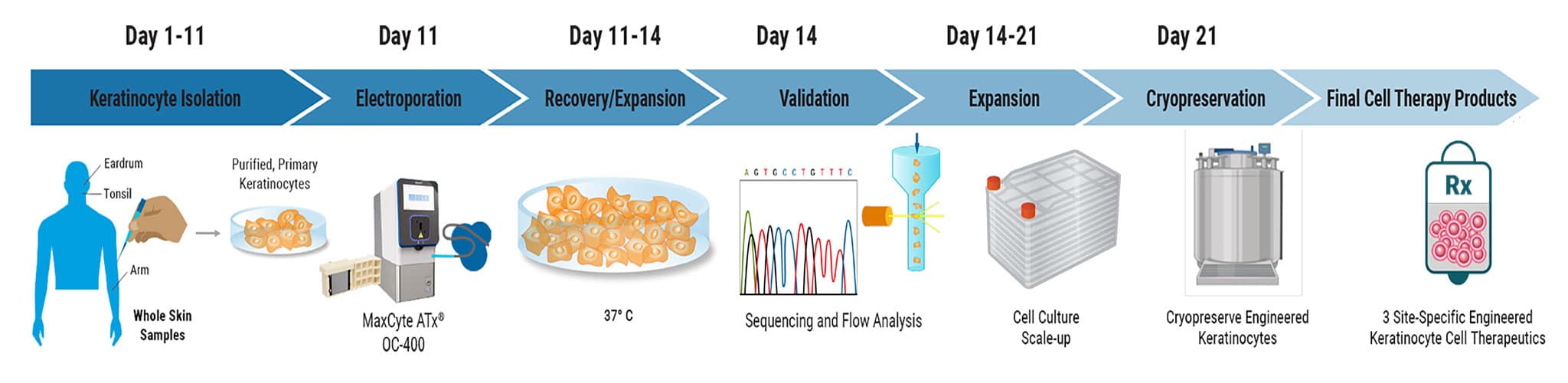
Summary of Keratinocyte Genome Engineering Process
High Efficiency Delivery of RNA and CRISPR RNPs to Primary Keratinocytes Using MaxCyte Electroporation

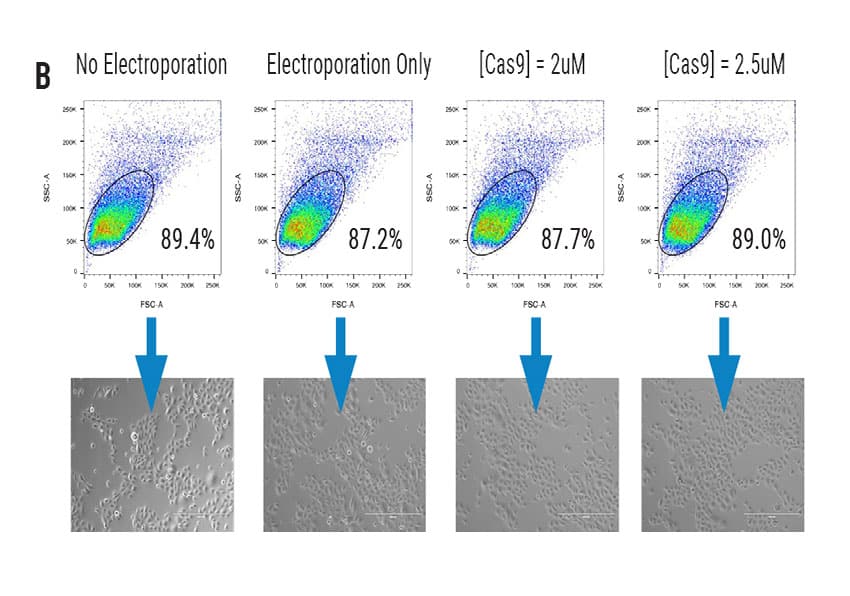
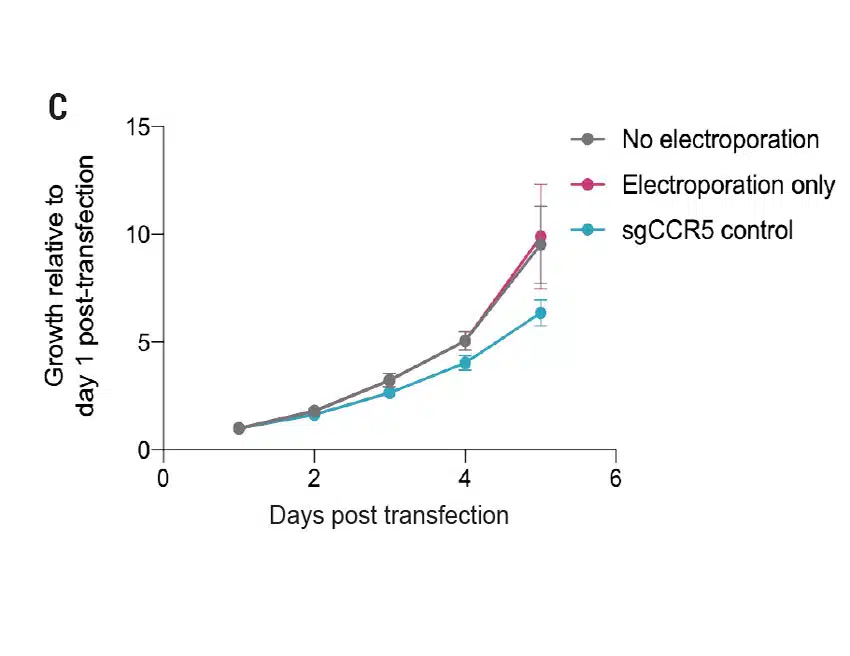
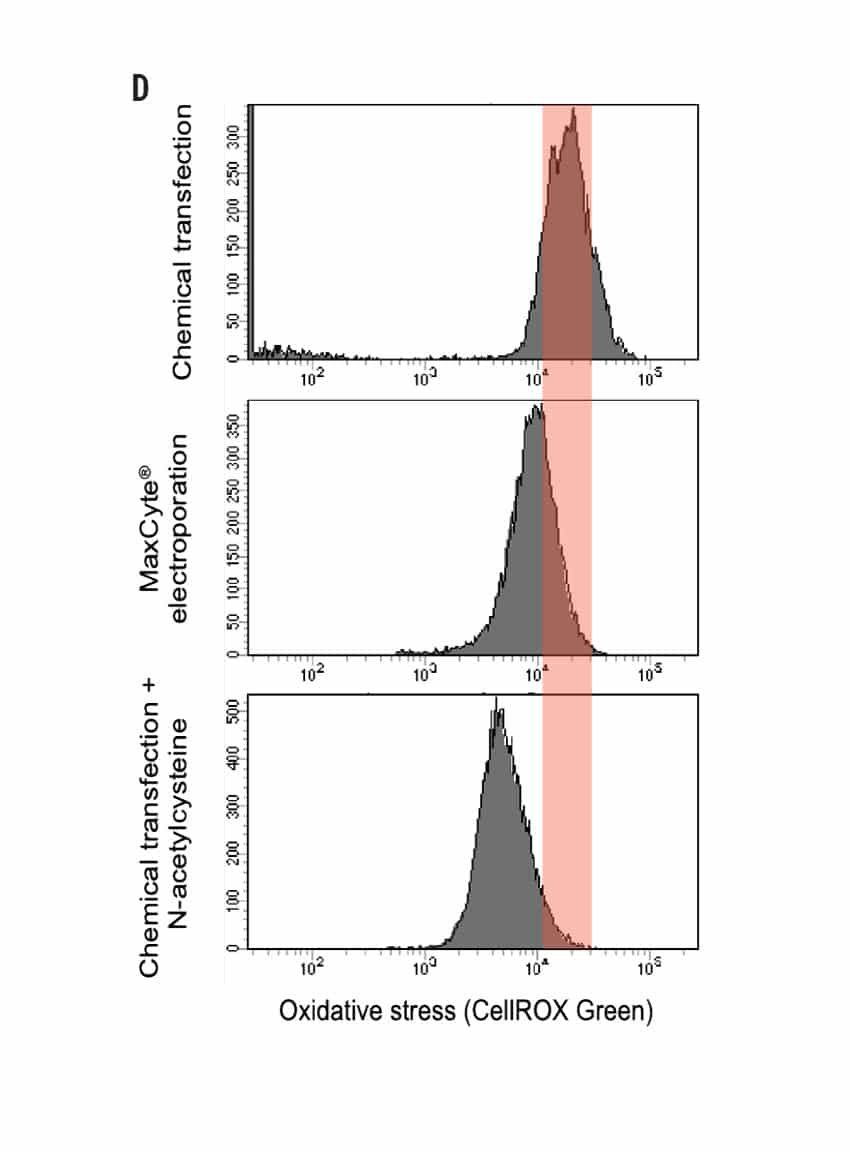
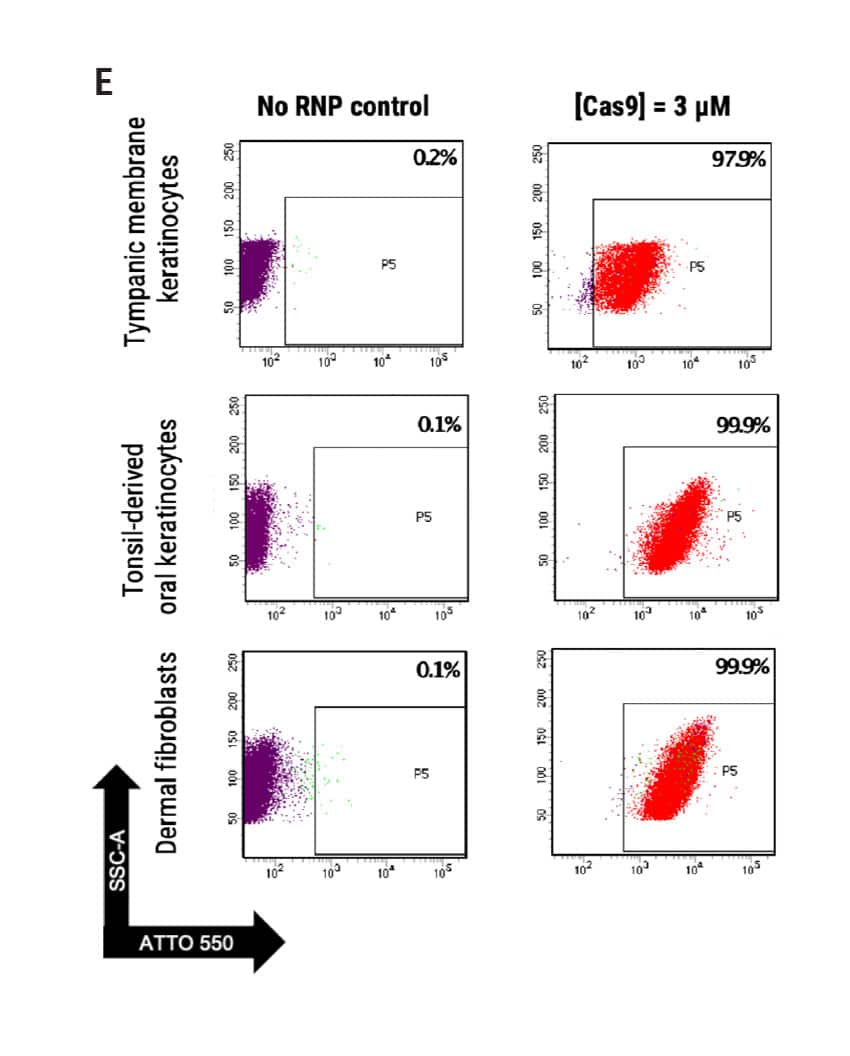
Figure 1 A) Primary foreskin keratinocytes were electroporated with mRNA-GFP or ATTO550-labeled CRISPR RNPs using the MaxCyte ATx® instrument and analyzed via flow cytometry 24 hours post transfection. B) Flow cytometry analysis of primary cell population and accompanying brightfield microscopy images (scale bar = 400 μM) of transfected keratinocytes from (A) 24 hours post electroporation. C) Growth profile of primary keratinocytes following electroporation with or without a CCR5-targeted CRISPR RNP. Growth measured via alamarBlueTM assay at indicated time points. D) Oxidative stress measured via CellROXTM Green assay in primary keratinocytes transfected via MaxCyte electroporation or standard chemical transfection. E) Primary dermal fibroblasts, tonsil-derived oral keratinocytes, and tympanic membrane (middle ear) keratinocytes were electroporated with ATTO 550-labeled CRISPR RNPs using the MaxCyte ATx instrument and analyzed via flow cytometry 24 hours post transfection.
Gene Knockout of Multiple Targets in Primary Keratinocytes Derived from Distinct Anatomical Sites
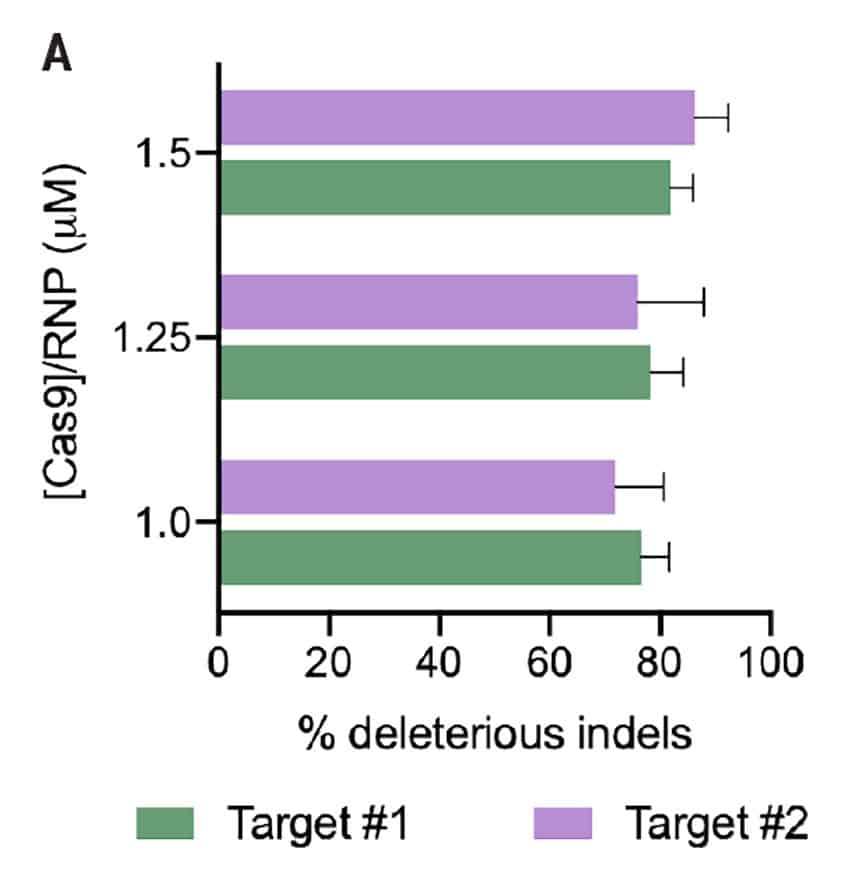
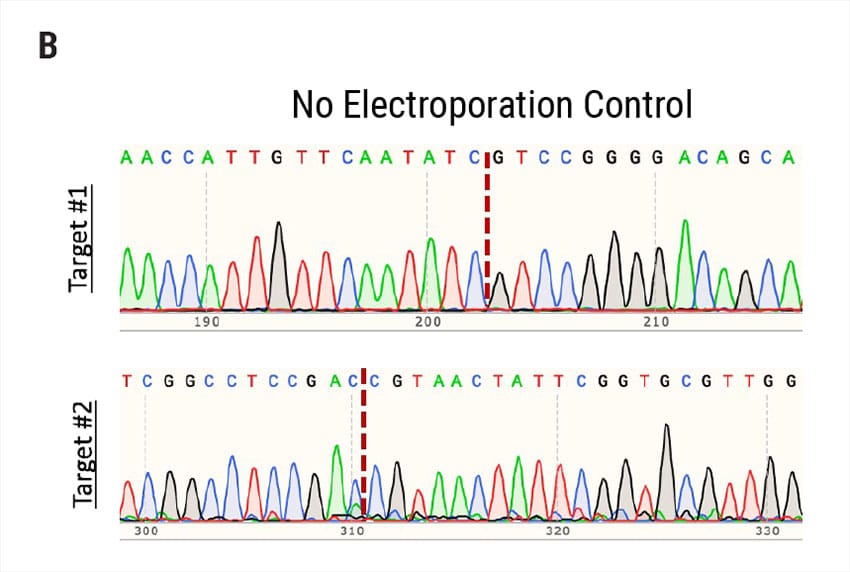
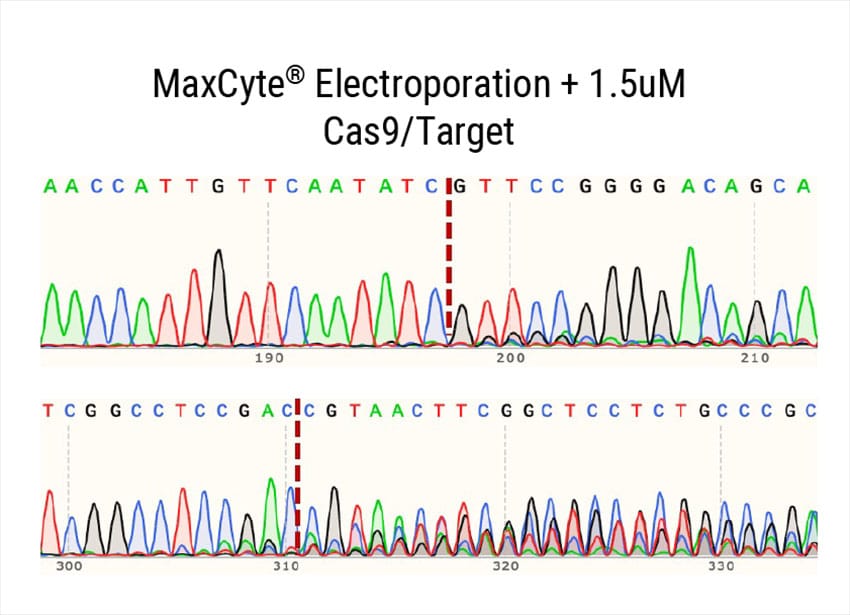
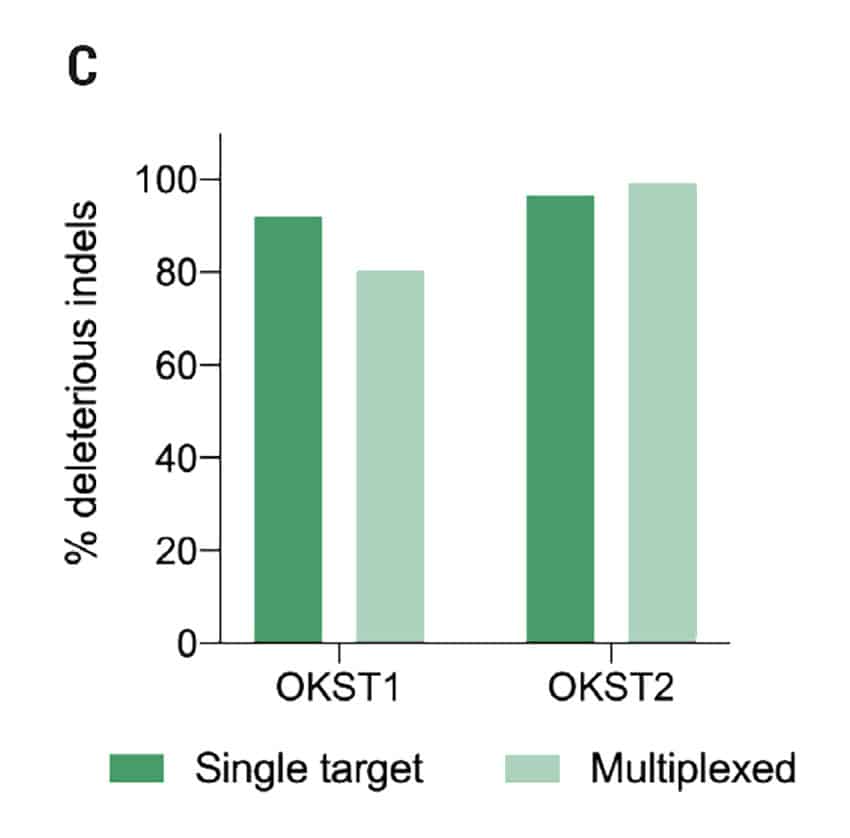
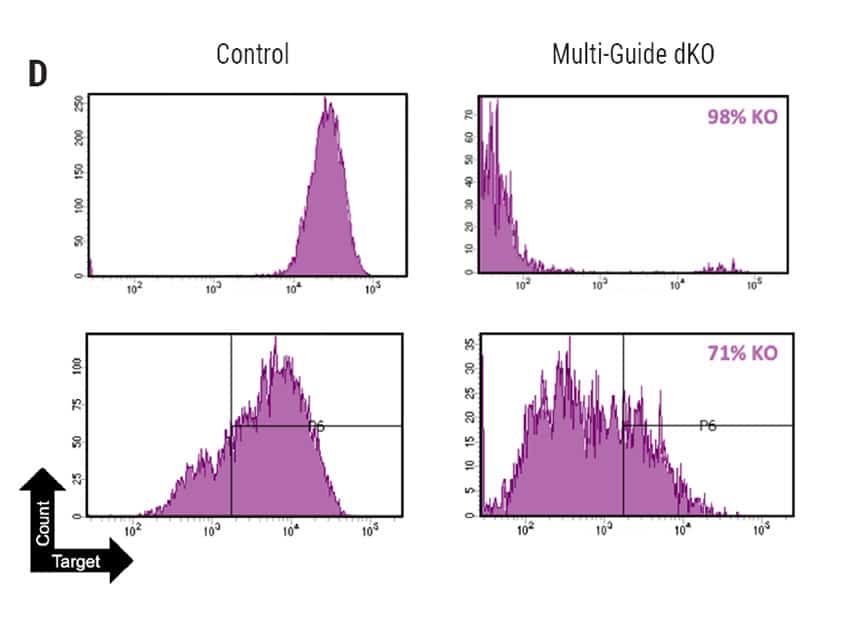
Figure 2: A) Percent deleterious indels measured via the TIDE assay 96 hours following electroporation of two CRISPR RNPs in primary foreskin keratinocytes. B) Representative Sanger sequencing traces from (A). C) Percent deleterious indels of two oral keratinocyte-specific targets (OKST1 and OKST2) measured via the TIDE analysis 96 hours following electroporation of either single or dual CRISPR RNPs in primary oral keratinocytes. D) Flow cytometry analysis of two distinct cell therapy targets 96 hours post electroporation of 6 CRISPR RNPs using the Synthego® multiguide gene knockout kit in primary forearm-derived skin keratinocytes
Adaptable Strategies for Development of a Clinical-Scale Keratinocyte Cell Therapy Manufacturing Process
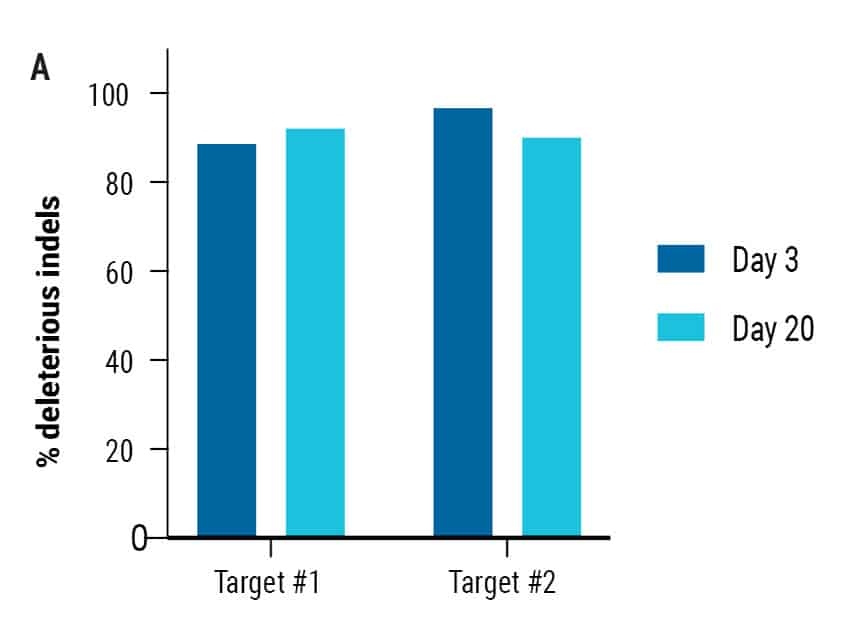
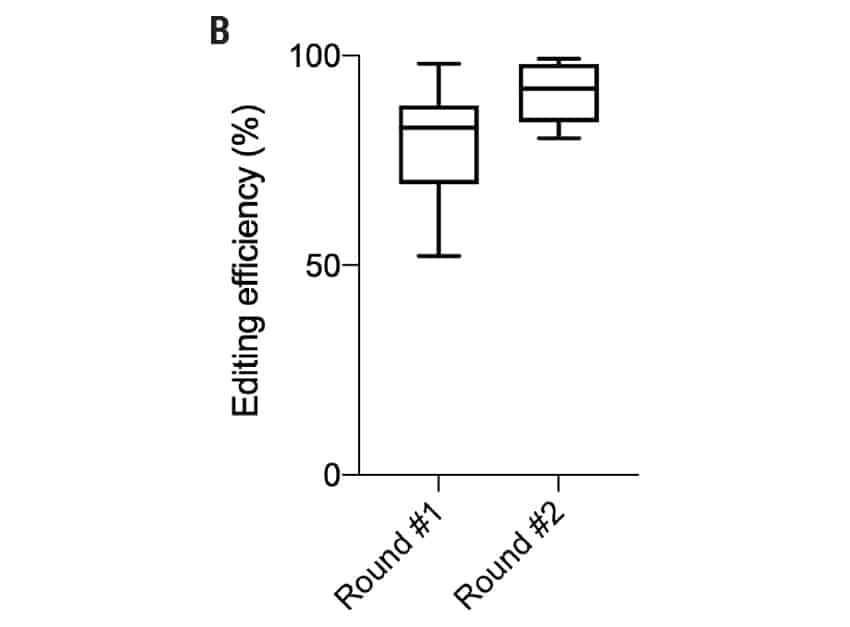

Figure 3: A) Percent deleterious indels were measured via TIDE analysis either 72 hours or 20 days following electroporation of two CRISPR RNPs in primary oral keratinocytes. B) Editing efficiency was measured via Sanger sequencing or flow cytometry in foreskin, oral, skin, and tympanic membrane keratinocytes following two rounds of electroporation-mediated gene editing C) Transfection efficiency (left) and editing efficiency (right) of 6 x 106 foreskin keratinocytes edited in a single round of electroporation.
MaxCyte Electroporation Enables Faster Keratinocyte Engineering
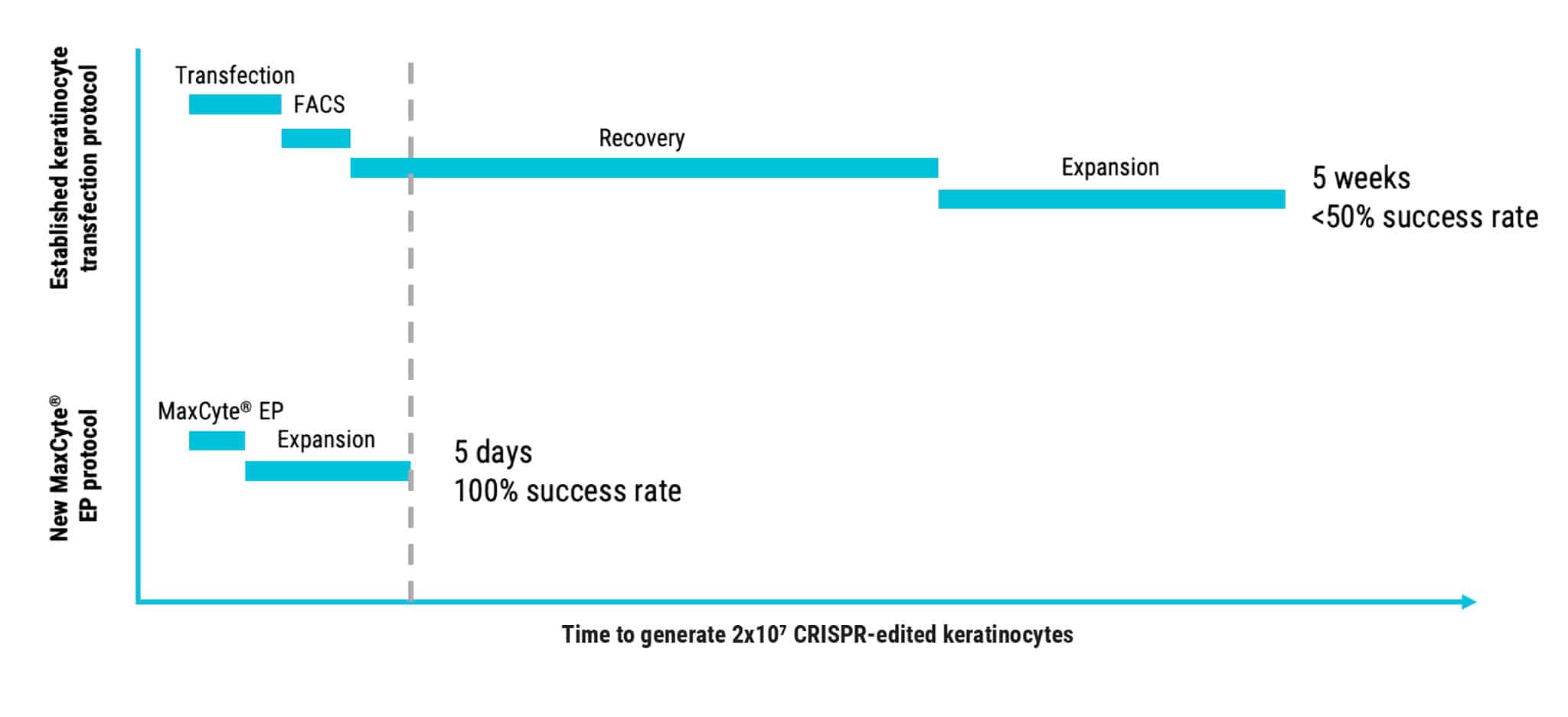
Figure 4: Faster primary keratinocyte genome engineering with reduced risk enabled by MaxCyte electroporation.
Summary
- Development of cell therapeutics using engineered keratinocytes has been limited by lack of options for efficient transfection.
- The MaxCyte ATx enables the efficient delivery of mRNA and CRISPR RNPs to hard-to-transfect primary keratinocytes, derived from multiple anatomical locations, in a scalable, GMP-compliant process.
- Electroporation maintains high viability, normal morphology, and low oxidative stress in primary neonatal and adult keratinocytes.
- MaxCyte electroporation enables delivery of up to 6 CRISPR RNPs, over multiple rounds of transfection, with no loss of efficiency.
- Platform flexibility allows for the rapid development of autologous and allogeneic cell therapy processes for specific subsets of keratinocytes and fibroblasts.
Corresponding Author: James Brady; [email protected] MaxCyte, Inc., Tel: (301) 944-1700





