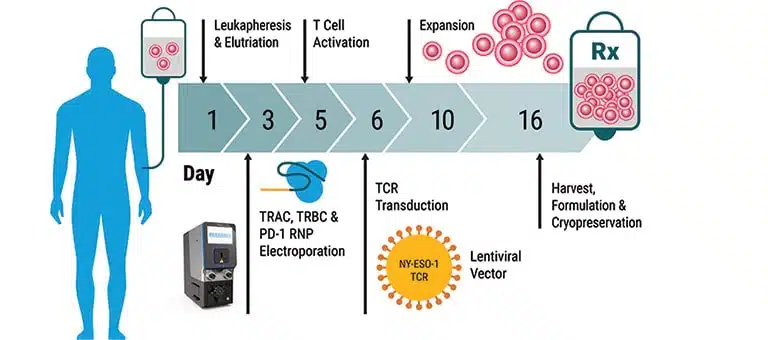
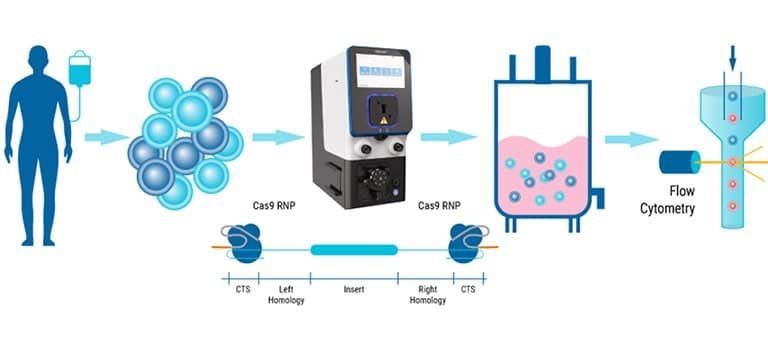
Electroporation Applications
Electroporation has evolved significantly over the years. Alternative methods were often limited by low efficiency or poor cell viability, but MaxCyte’s advanced electroporation platform overcomes these challenges. Our proprietary technology enables high-performance transfection across a variety of applications, from complex gene editing to large-scale transient protein production.
Research applications
Electroporation is more than just a transfection method—it’s a cornerstone of modern biotechnology and molecular biology. Its ability to deliver therapeutic molecules, genetic material and small compounds into cells makes it indispensable for developing gene-edited cell therapies, producing monoclonal antibodies and biologics and exploring novel drug targets in preclinical research, among other applications. Learn how electroporation can support your specific research area below.
Challenges in electroporation & how MaxCyte® solves them
Transfecting difficult cell types
Primary cells, stem cells and certain immune cells are notoriously hard to transfect with traditional methods. MaxCyte’s platform delivers superior results, ensuring high viability and efficiency even for challenging cell types.
Scaling up from R&D to manufacturing
Many electroporation platforms fail to scale effectively for clinical and commercial applications. MaxCyte’s scalability allows seamless transitions across research, preclinical and manufacturing workflows.
Regulatory requirements for cell therapies
Navigating compliance for cell and gene therapies can be daunting. MaxCyte’s technology is GMP-compliant and supported by a history of regulatory approvals, ensuring smooth product development and commercialization.
Electroporation in emerging fields
Stay ahead in research and development with electroporation applications tailored to cutting-edge industries:
- Synthetic biology: Deliver plasmids and genetic constructs into cells for creating engineered organisms.
- mRNA therapeutics: High-efficiency delivery of mRNA for vaccine development and therapeutic applications.
- Functional genomics: Accelerate gene knockout or knockin studies with precise CRISPR delivery.
Ready to learn how MaxCyte enables your electroporation applications?
Answers are just a click away!