Poster
Highly Efficient Engineering of Difficult-to-Transfect Immune Cells Using MaxCyte® Electroporation
Abstract
Since their inception, cell-based therapies have emerged as promising treatments for a wide range of diseases. Immune cells such as T cells, NK cells and macrophages are being used to treat various cancers, autoimmune disorders and degenerative diseases. To engineer these cells for improved efficacy and safety, biomolecules and other genome-editing tools must be delivered into these difficult-to-transfect cells. To this end, MaxCyte has developed optimized cell engineering workflows using the ExPERT™ electroporation platform that enable highly efficient delivery of molecules, such as RNA, DNA and CRISPR-Cas nucleases, into a variety of cell types. Here, we demonstrate that these workflows can be used to engineer primary human immune cells to express tumor-targeting receptors while maintaining high cell viabilities and functionality. In particular, MaxCyte enabled transient and stable expression of CARs/TCRs in T cells, NK cells and macrophages through high-efficiency transfection of mRNA, DNA encoding transposons/transposases, or CRISPR ribonucleoproteins (RNPs) and homology-directed repair (HDR) templates into these hard-to-transfect cells. In addition, these workflows seamlessly scaled up, allowing these cells to be engineered at therapeutically relevant scales.
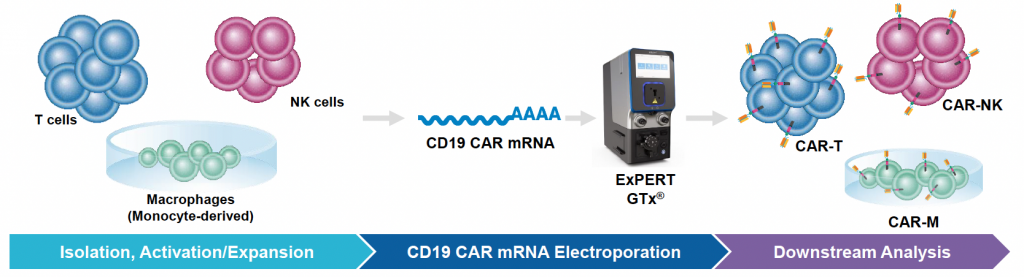
CD19 CAR mRNA engineering of primary immune cells using MaxCyte electroporation
A) Workflow with ExPERT GTx®
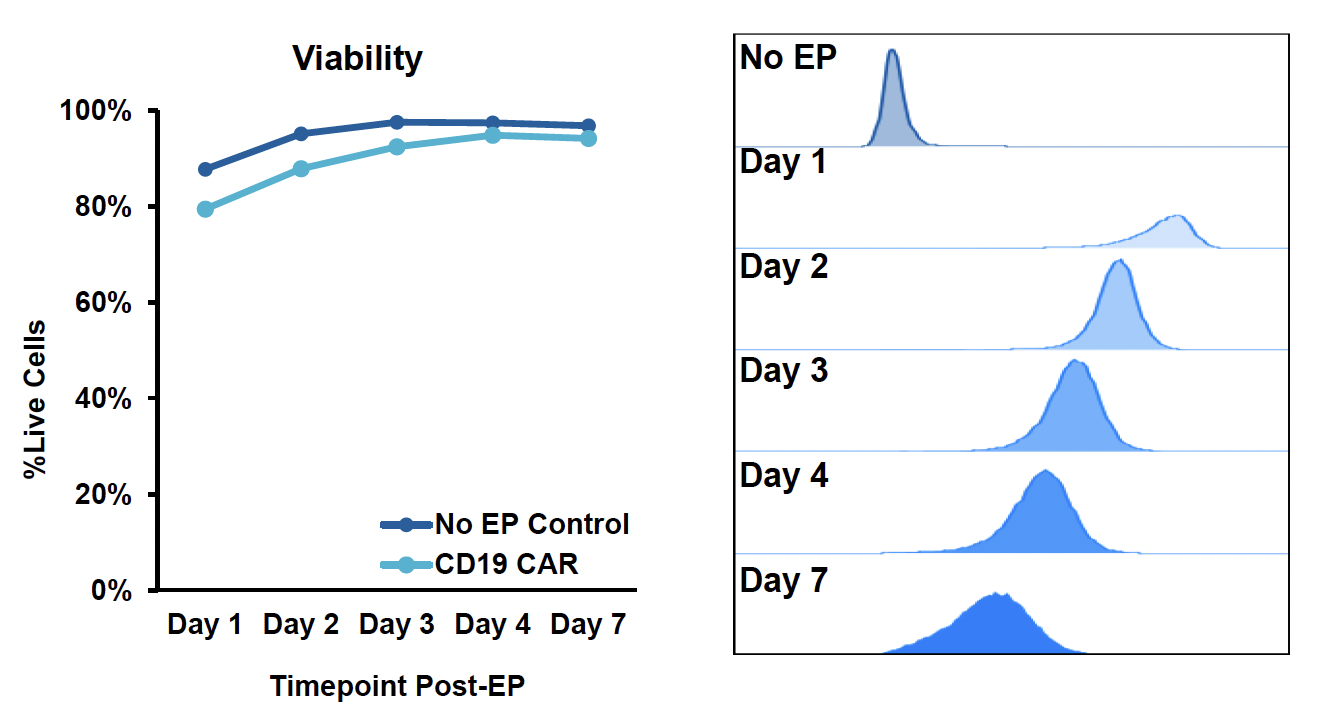
B) Transient CAR expression in T cells
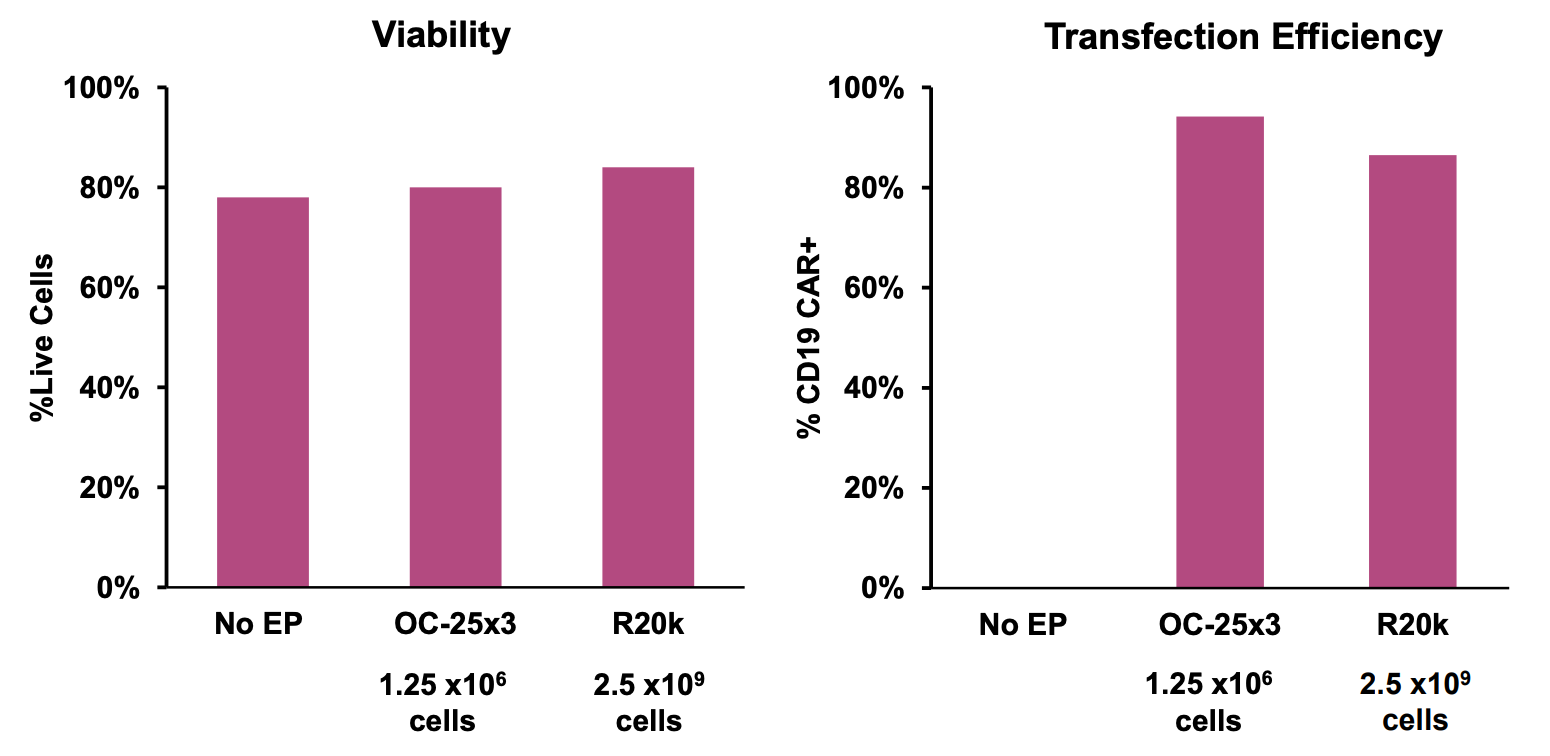
C) CAR mRNA engineering of primary NK cells
D) CAR mRNA engineering of monocyte-derived macrophages
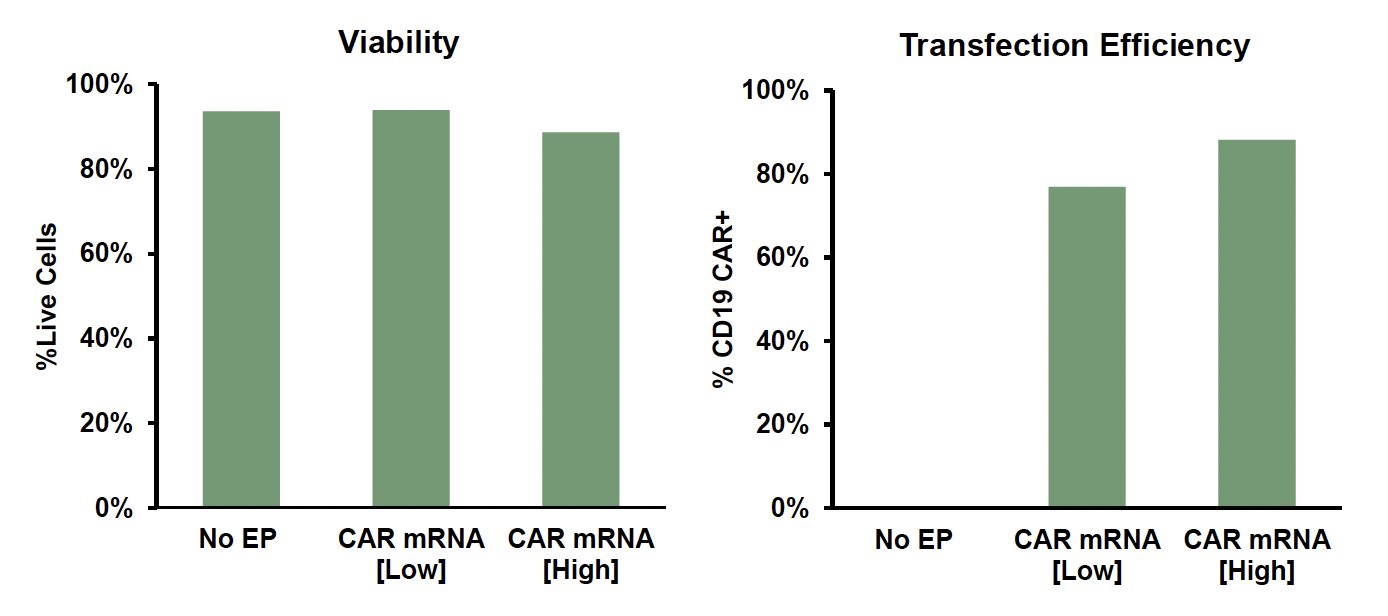
Figure 1: A) General workflow for engineering primary human immune cells with CD19 CAR mRNA electroporated using the ExPERT GTx®. B) T cells were isolated and activated prior to transfection. CD19 CAR-T cells were expanded, and transfection efficiency and cell viability was examined over a 7-day period after electroporation (EP) by flow cytometry. C) NK cells were isolated and expanded for 14 days using a feeder-free culture method. Expanded NK cells were electroporated using either a small-scale OC-25x3TM (static) processing assembly or a large-scale R20k (flow) processing assembly. Transfection efficiency and cell viability were examined 24h post-EP by flow cytometry. D) Monocytes were isolated and differentiated into macrophages for 7 days and subsequently transfected with either a high or low concentration of CD19 CAR mRNA. Transfection efficiency and viability were examined 24h post-EP by flow cytometry.
Engineering TCR-T cells for immunotherapy of hepatocellular carcinoma
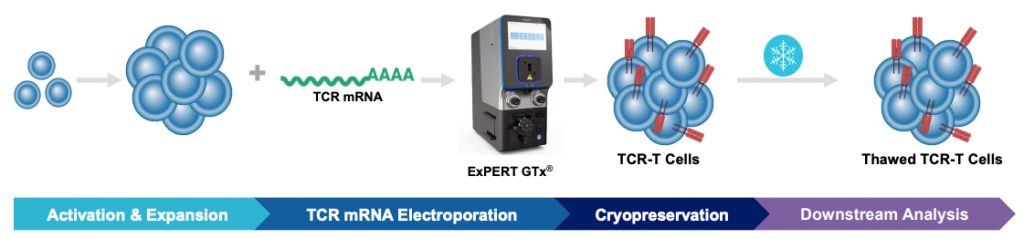
A) Workflow with ExPERT GTx
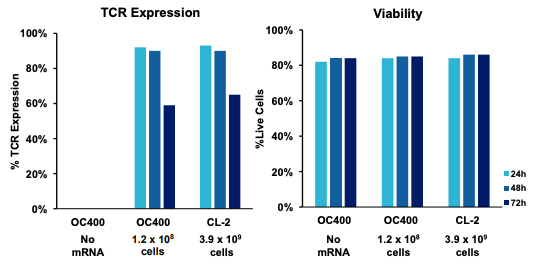
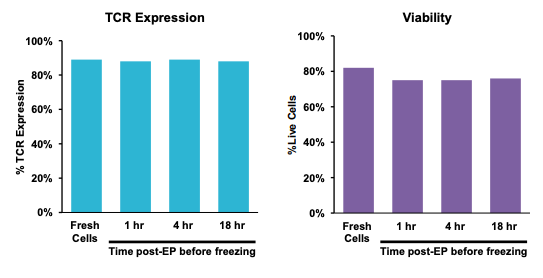
B) TCR expression and viability by processing assembly and time post electroporation
C) TCR expression and viability over culture time
D) IFNγ expression over time
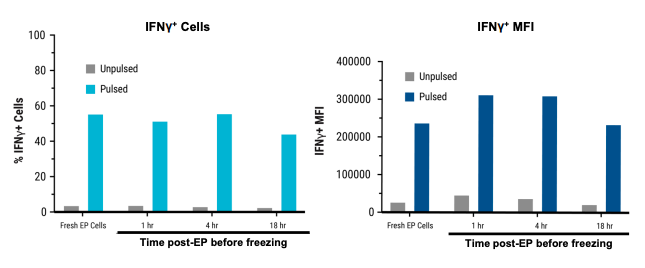
Figure 4: A) Schematic workflow of primary activated human T cells electroporated with TCR mRNA using an ExPERT GTx®. B) Primary activated T cells were electroporated with TCR mRNA in MaxCyte static (OC-400TM) or flow (CL-2TM) processing assemblies (PAs). The total number of T cells electroporated in the OC-400 and CL-2 was 1.2x108 and 3.9x109, respectively. T cells were analyzed 24, 48 and 72 hours after transfection by flow cytometry. C) Electroporated cells were divided into four groups. The first group was cultured for 24 hours at 37°C and analyzed by flow cytometry. The remaining three cell groups were cultured at 37°C for one, four and 18 hours after transfection, and then cryopreserved. After thawing, these three cell groups were cultured and then analyzed for TCR expression and viability. D) TCR expressing lymphocytes were co-cultured with antigen peptide-pulsed T2 cells and analyzed by flow cytometry for IFNγ expression.
This content was reproduced with the permission of Lion TCR.
Engineering CD70-directed CAR NK cells for treatment of hematological and solid malignancies
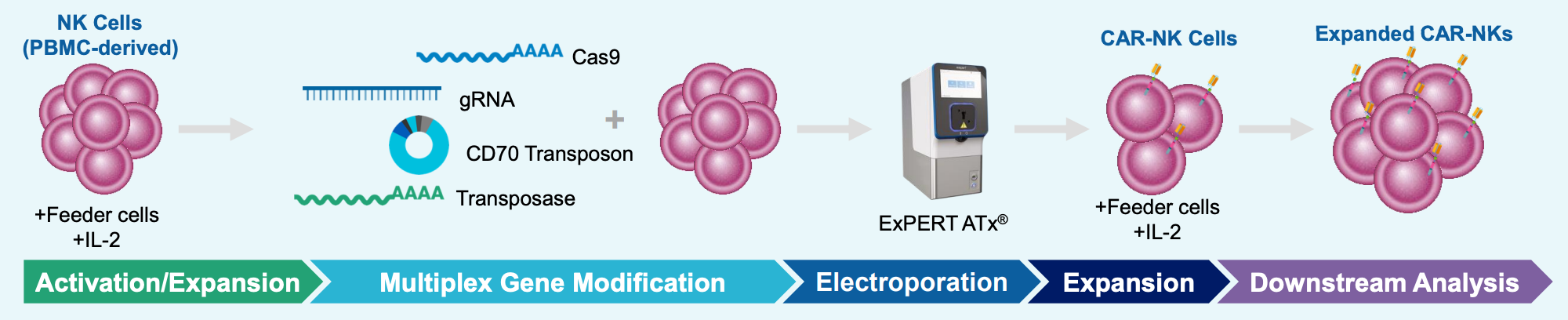
A) Workflow with ExPERT ATx®
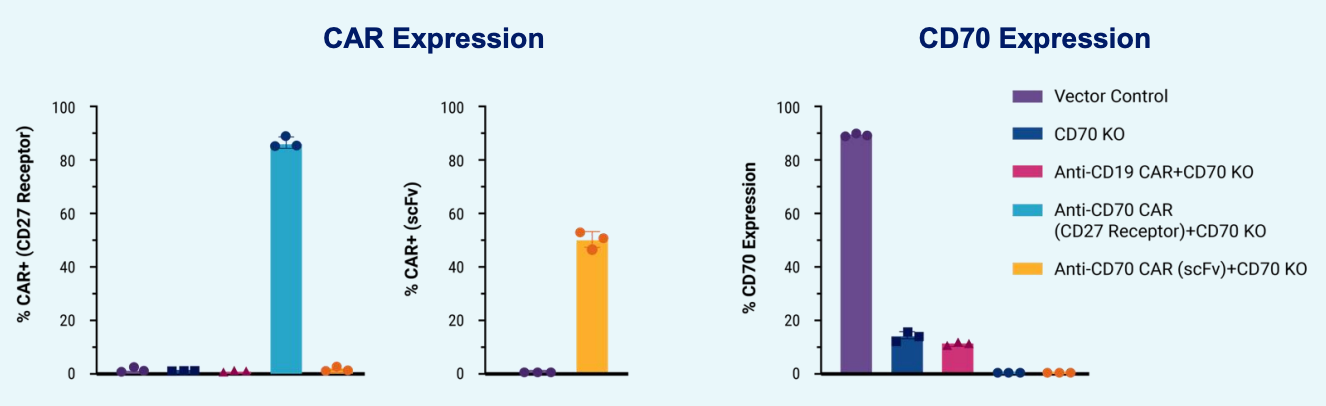
B) CAR expression in engineered NK cells compared to controls
C) Cytotoxicity, degranulation and cytokine expression after co-culturing of engineered NK cells with target cells
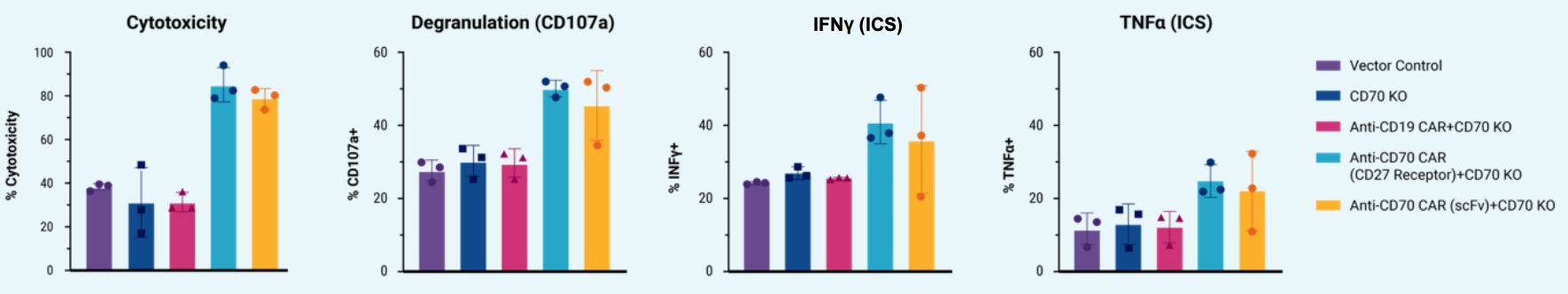
Figure 2: A) Schematic workflow of primary human NK cells electroporated with TcBuster™ transposase-mRNA, CD70 CAR transposon plasmid DNA, mRNA-encoding Cas9 and a CD70 sgRNA using an ExPERT ATx®. Primary NK cells were co-cultured with engineered feeder cells and IL-2. B) CAR expression in engineered NK cells was characterized with either anti-CD27 antibody (CD27 receptor detection) or recombinant CD70 protein (scFv detection). Controls include plasmid only, CD70 KO only and anti-CD19 CAR+CD70 KO. All data points represent individual donors. C) Engineered NK cells were co-cultured with luciferase-expressing CD70-positive NOMO-1 (AML) target cells; cytotoxicity, degranulation and cytokine expression were characterized. All data points represent individual donors, and error bars represent standard deviation.
This content was reproduced with kind permission from Catamaran Bio Inc.
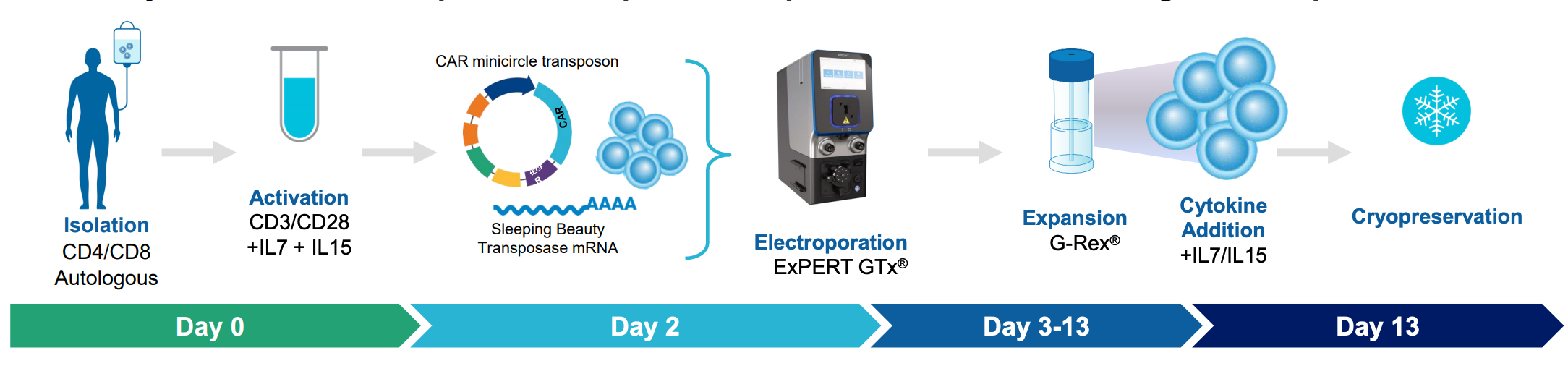
MaxCyte enabled development of rapid and reproducible manufacturing of TranspoCART cells
A) Workflow with ExPERT GTx
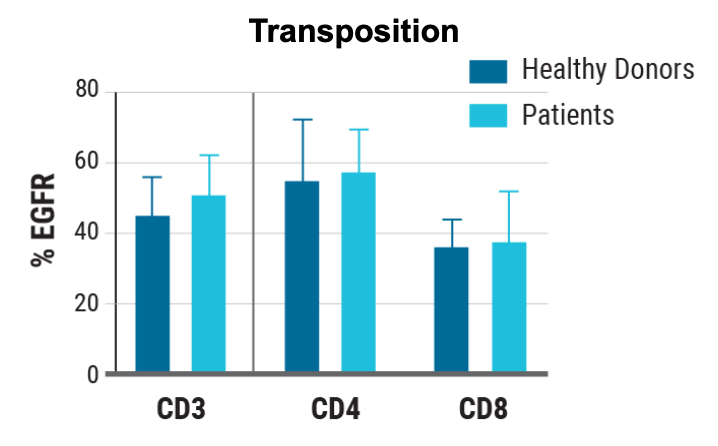
B) Rate of transposon insertion
C) TranspoCART expansion

B) TCR expression and viability by processing assembly and time post electroporation

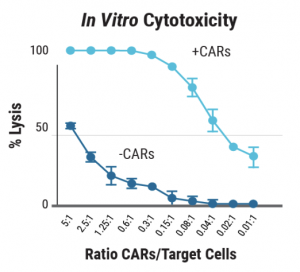
D) In vitro cytotoxicity
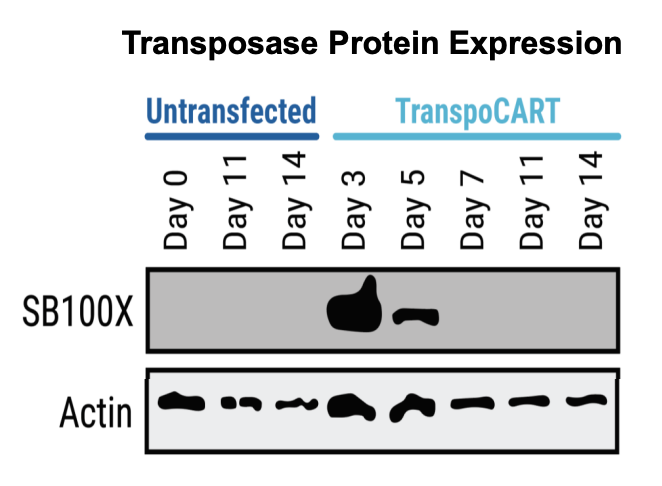
E) Western blot analysis
B) Quantitative PCR
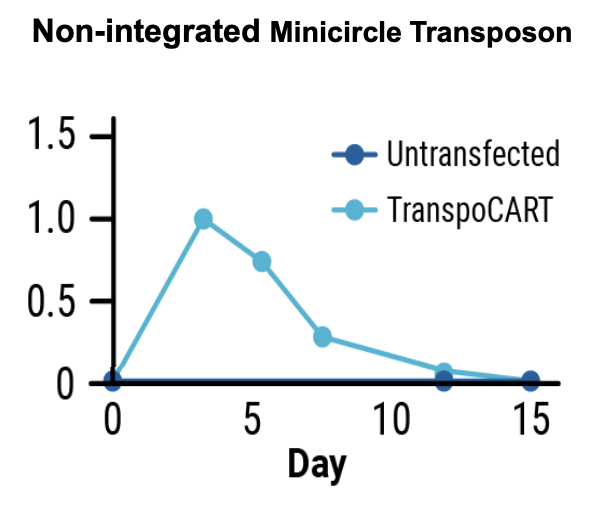
Figure 3: A) Primary human T cells were electroporated with Sleeping Beauty 100X mRNA transposase and transposon minicircle DNA encoding a CAR and truncated human EGFR (tEGFR). Expression of tEGFR was used as a marker for successful transposon insertion and as a safety switch, enabling TranspoCART cells to be targeted and depleted on treatment with anti-tEGFR antibodies. No differences in B) the rate of transposon insertion or C) TranspoCART expansion were seen between healthy donor and patient samples. D) TranspoCART cells from patients showed in vitro cytotoxicity against a CAR target-expressing cell line. E) Western blot analysis and F) qPCR showed transposase protein and unintegrated minicircle transposon DNA were undetectable by days seven and 15 after electroporation.
Data generated in collaboration with CIMA/Clinica Universidad de Navarra and CIEMAT. Adapted with permission.
CRISPR RNP and ssDNA co-electroporation in activated T cells for efficient CAR knockin
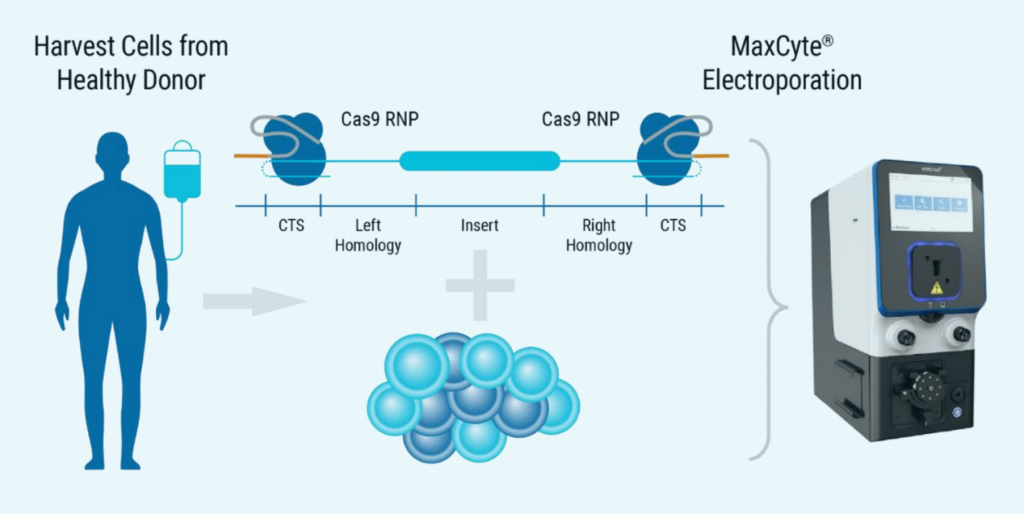
A) Visual summary of ExPERT GTx co-electroporation
B) KI efficiencies of BCMA-CAR days 7 and 10 of manufacturing

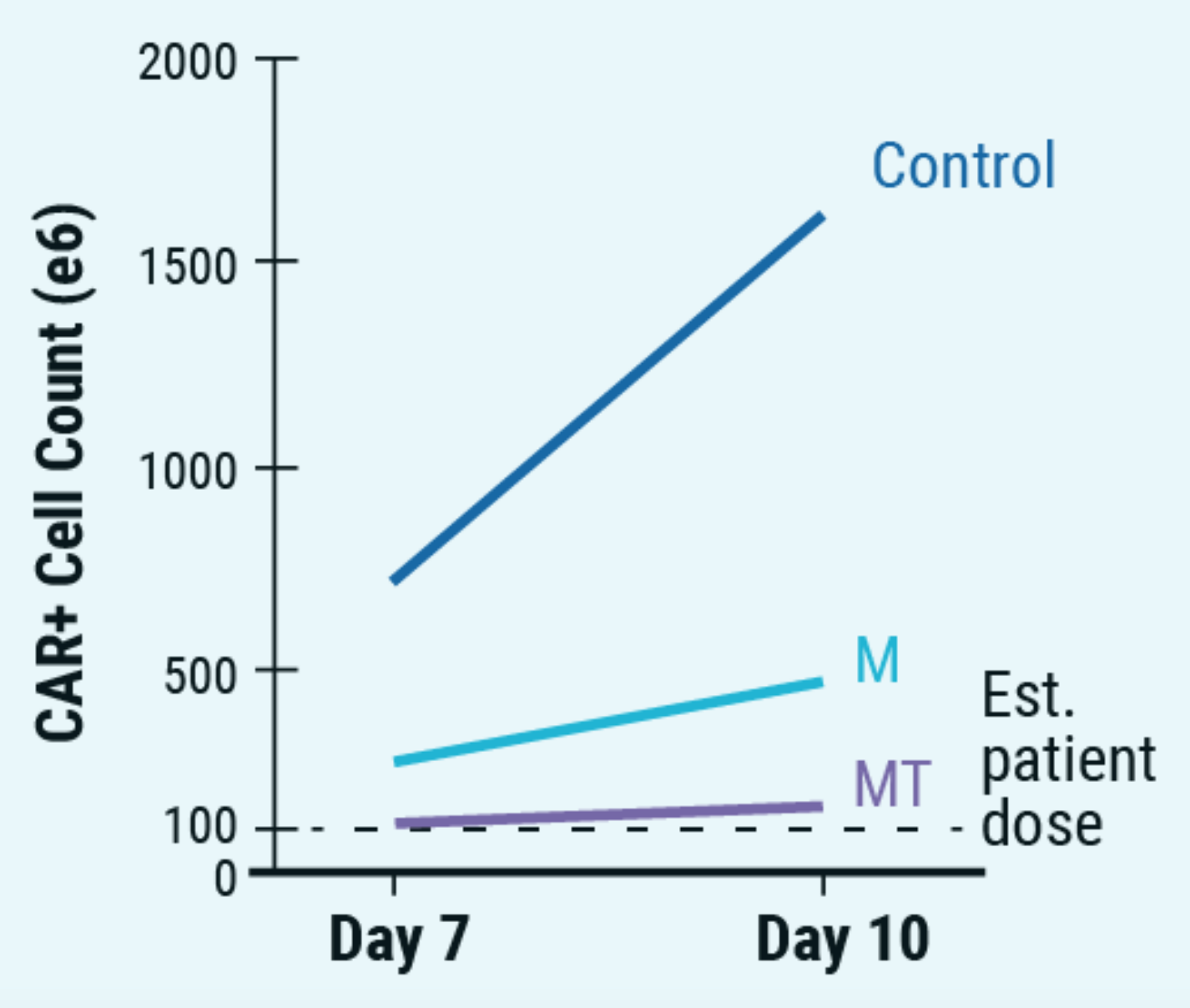
C) Number of CAR+ T cells on days 7 and 10 of manufacturing
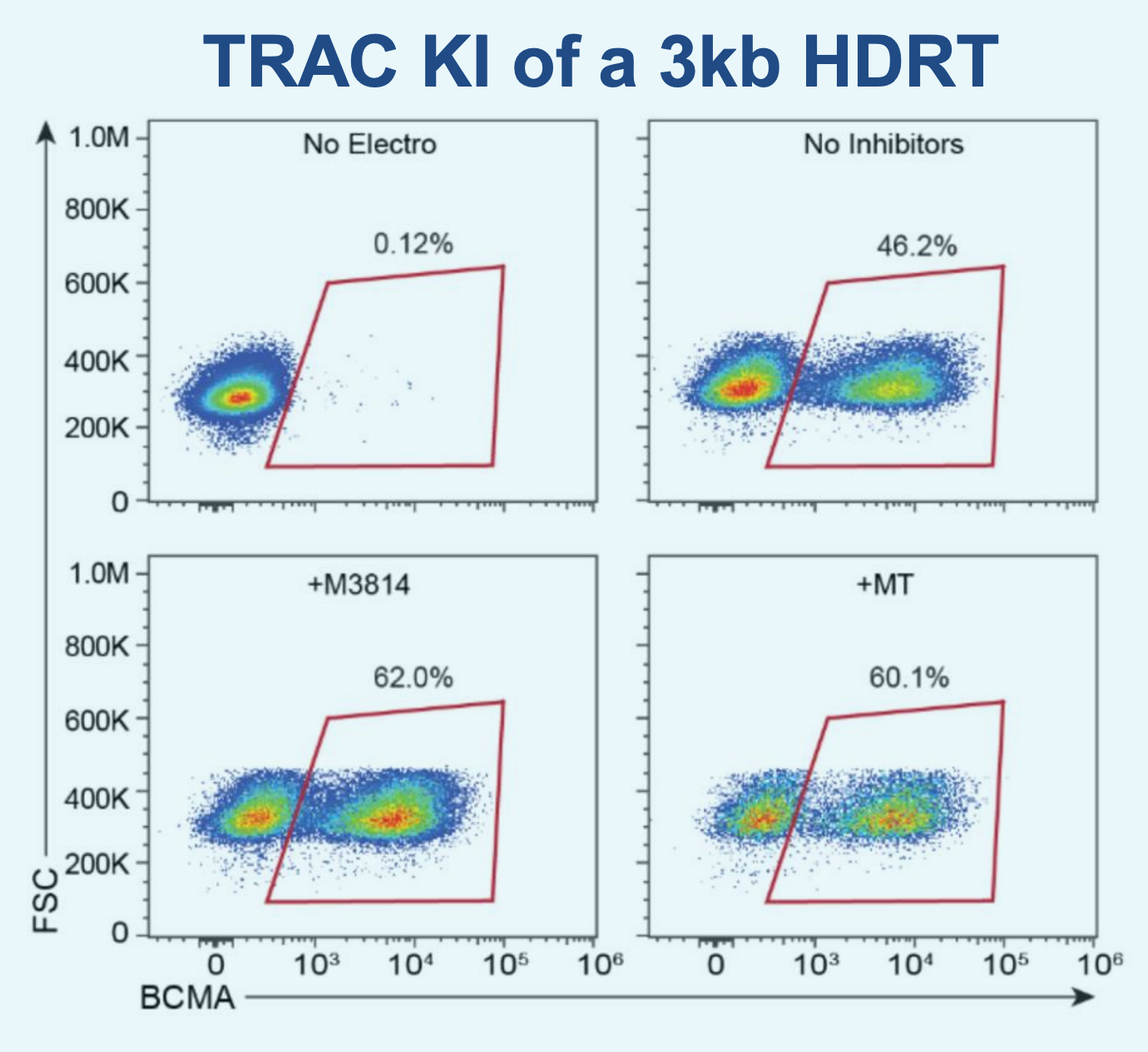
D) Flow plots of BCMA-CAR KI efficiencies
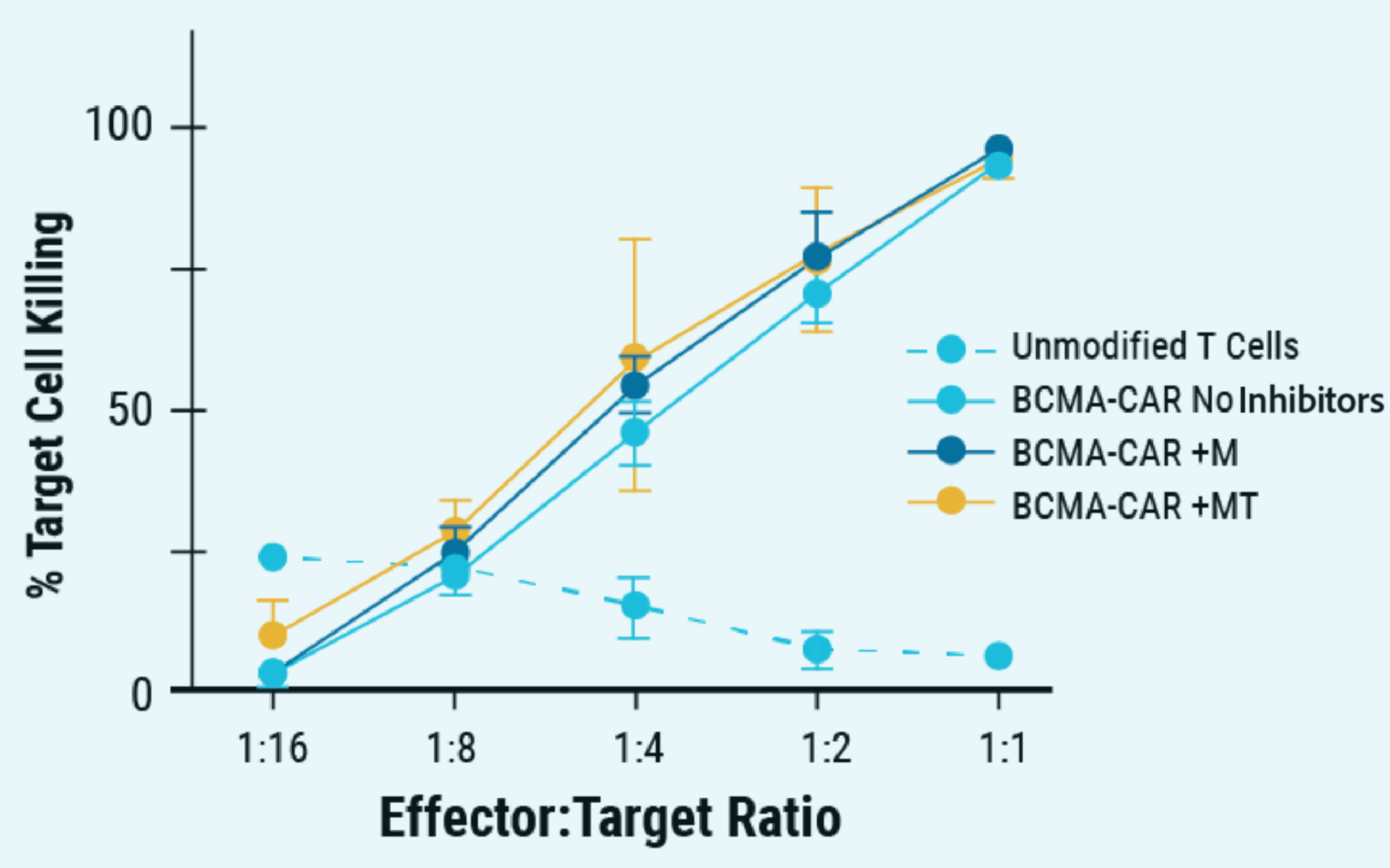
E) Percent target of myeloma cell killing over effector target ratio
F) Overall survival over day post tumor seeding when treated
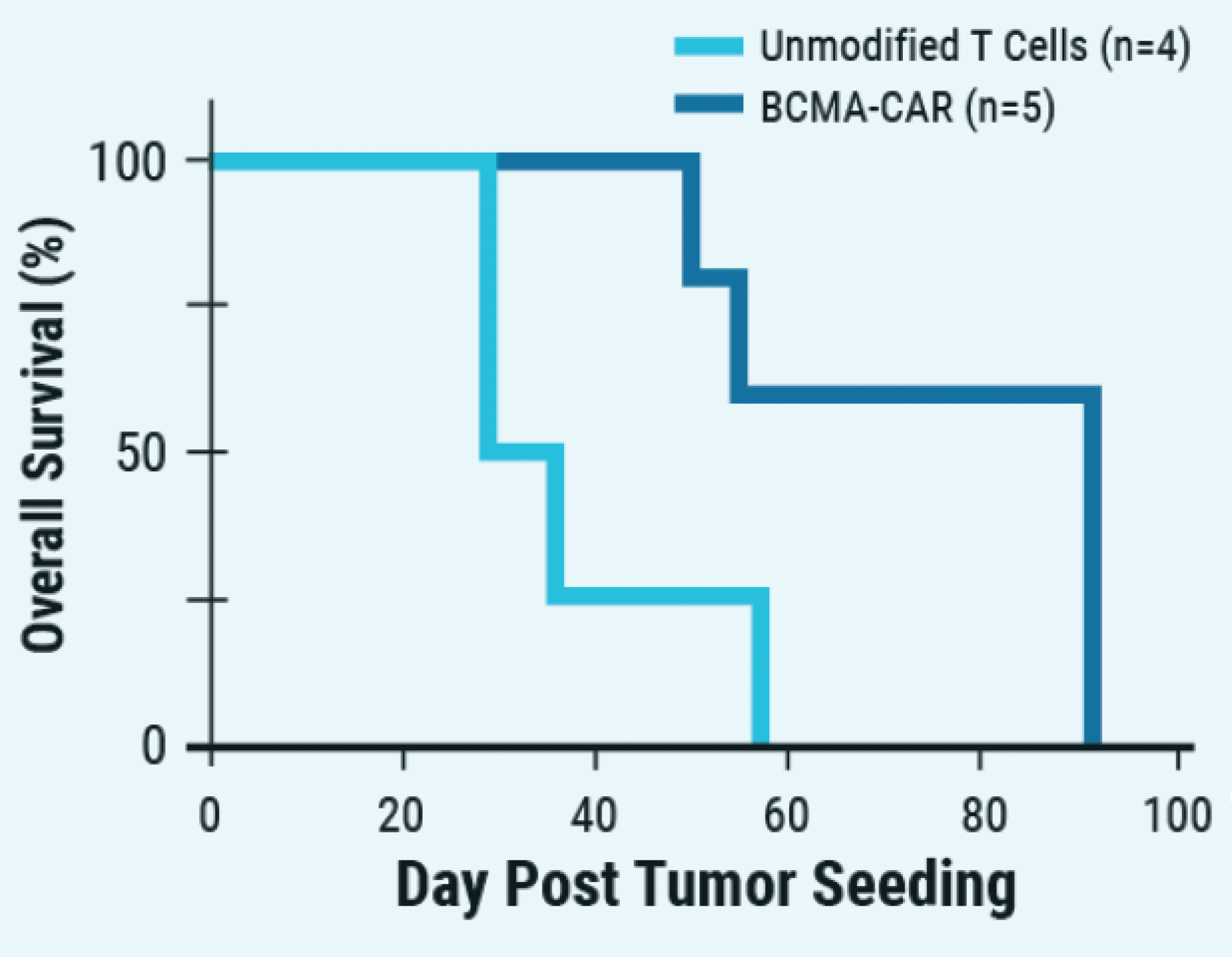
Figure 5: A) ExPERT GTx® electroporation was used to deliver CRISPR RNP + ssCTS template to the TRAC locus of activated T cells. B) KI efficiencies of BCMA-CAR on day seven and 10; Ctrl - no inhibitor, M - M3814M DNA-PK inhibitor and MT - M + histone deacetylase class I/II Inhibitor. C) The total number of CAR+ T cells on days seven and 10 of the cGMP manufacturing protocol. Dotted line - estimated patient dose of 1x108 cells. D) Flow plots of BCMA-CAR KI efficiencies for each condition on day 10. E) BCMA-expressing multiple myeloma cell lines were effectively targeted and killed by engineered CAR T cells; Effector: Target, 1:1. F) Overall survival was improved in tumor-bearing mice when treated with engineered CAR T cells or unmodified T cells from the same donor.
This content was adapted from Shy BR., Nat Biotechnol. 2023 Apr;41(4):521-531. doi: 10.1038/s41587-022-01418-8. PMID: 36008610.
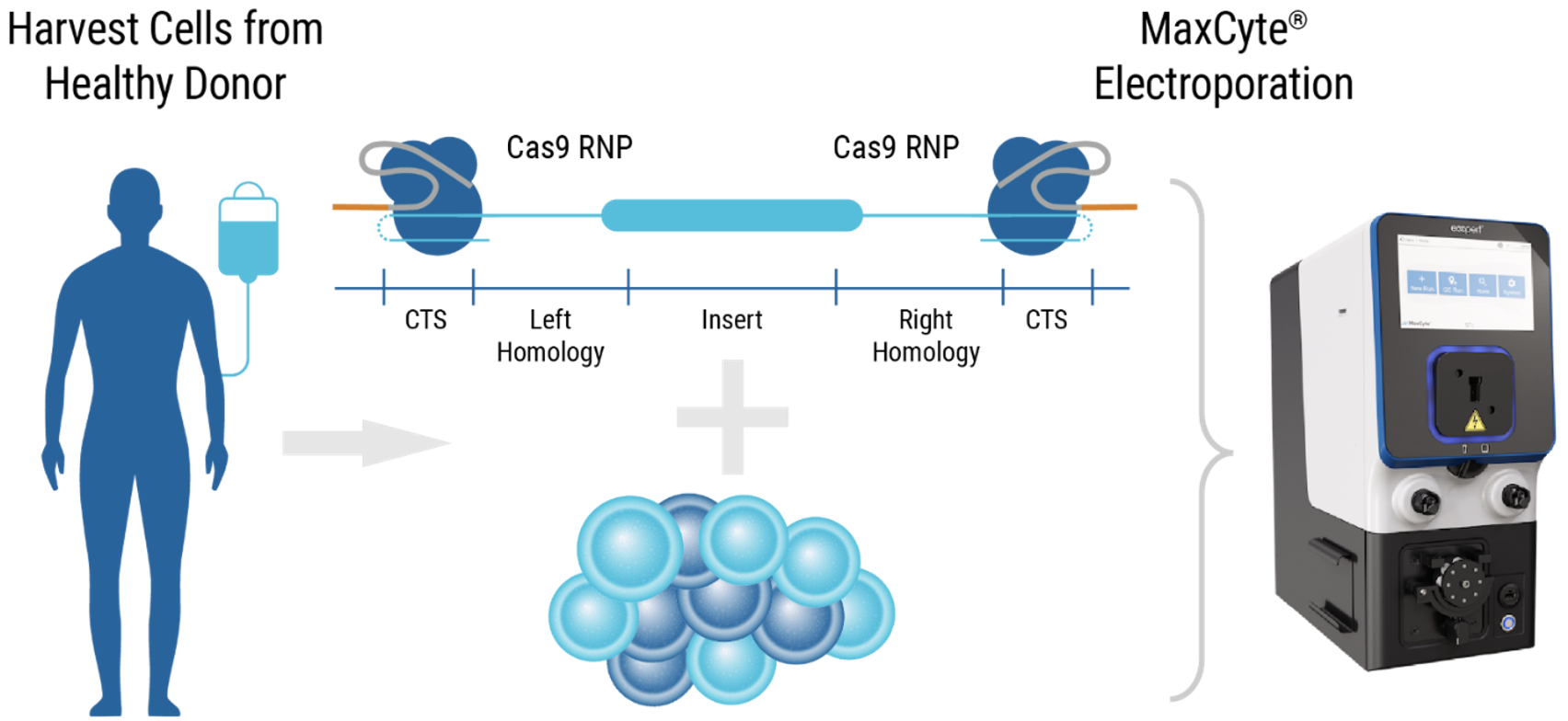
MaxCyte electroporation of dsDNA HDRT enables efficient knockout/knockin in human T cells
A) Workflow with ExPERT GTx
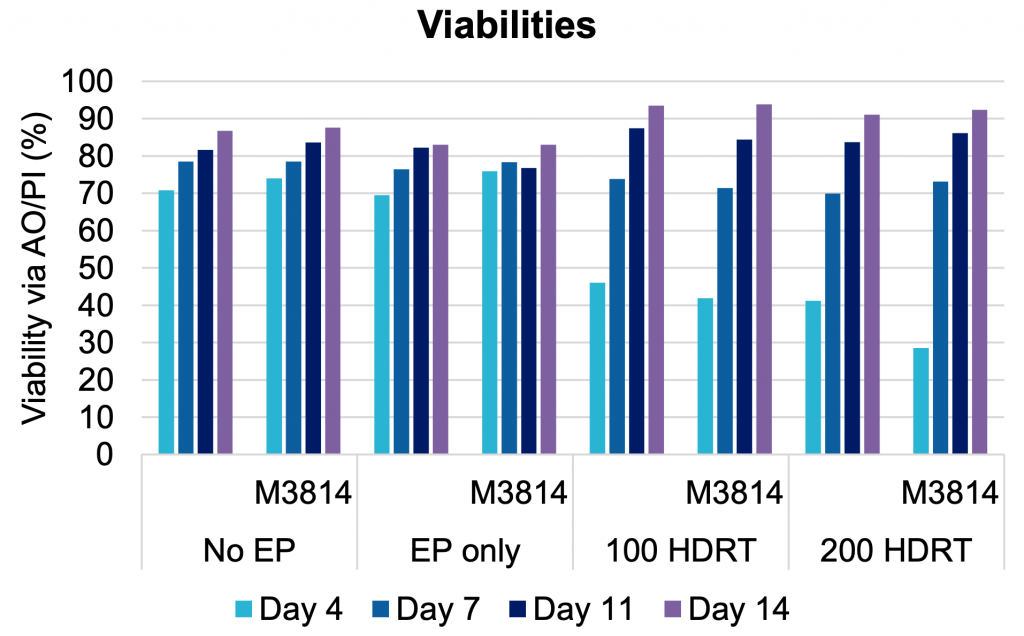
B) Cell viability over time
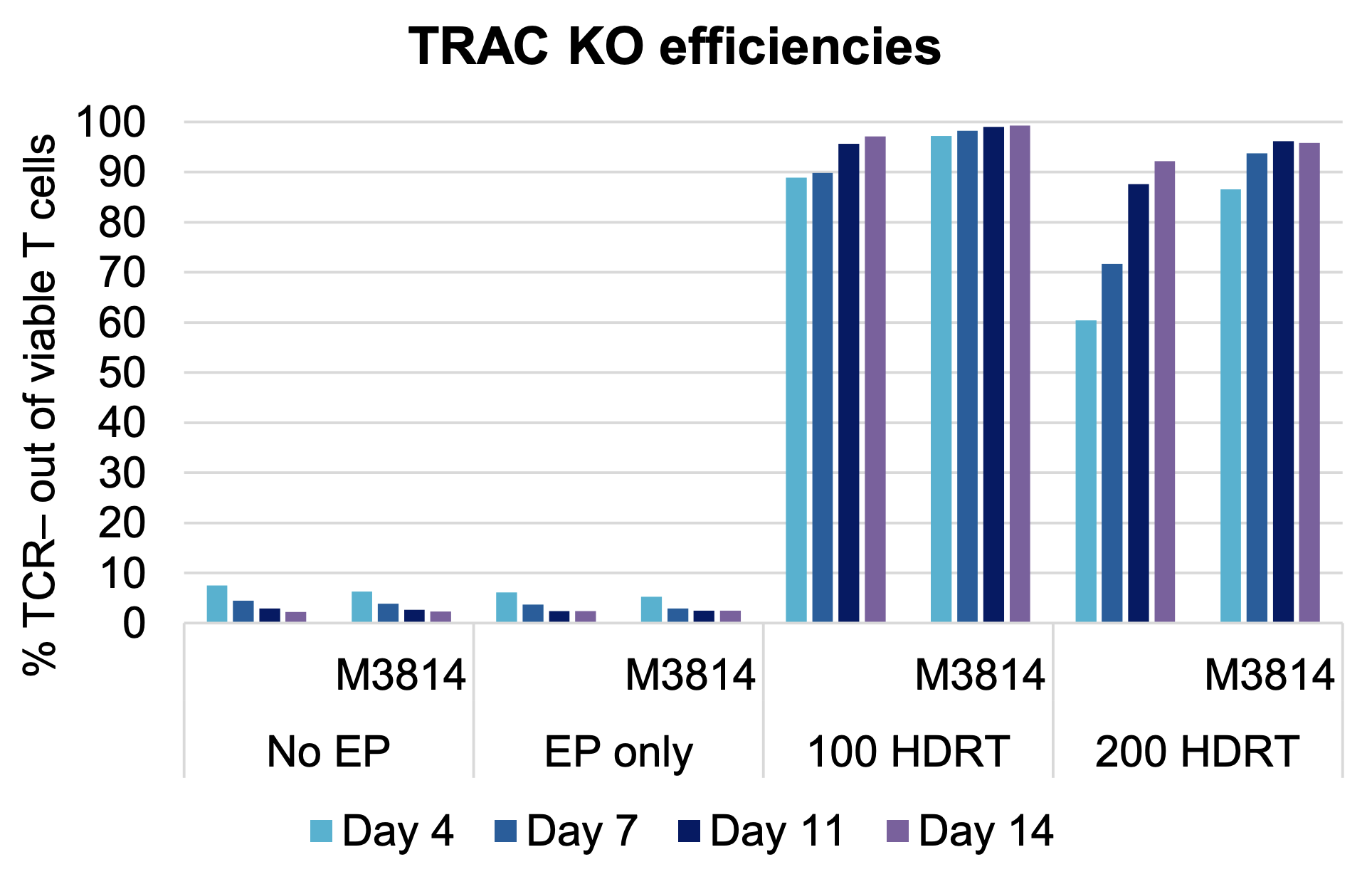
C) TRAC knockout efficiencies over time
D) CAR knockin efficiencies over time

Figure 6: A) Activated primary human T cells were electroporated with CRISPR RNP targeting the TRAC locus and two concentrations of a CD19 CAR HDRT in an OC-25x3 (3x25 mL) processing assembly. After resting, electroporated cells were cultured for 20 hours in media with or without M3814 enhancer (1 µM). Following a media change, cells were expanded for 14 days. (B) Cell viability, (C) TRAC knockout efficiency and (D) CAR knockin were measured periodically. Cell viability remained high from day seven to day 14 in all conditions tested. The addition of M3814 had no impact on cell viability or TRAC efficiency. Optimal CAR knockin was seen in the presence of enhancer.
Summary
MaxCyte electroporation can efficiently engineer primary human immune cells to express tumor targeting receptors while maintaining high cell viabilities and functionality.
- MaxCyte enabled transient expression of CARs/TCRs in T cells, NK cells and macrophages through highly efficient transfection of mRNA.
- Stable expression of CARs in T cell and NK cells was accomplished by efficient integration of DNA-encoding transposon systems that showed both high viability and cytoxicity against target cells.
- T cell engineering using CRISPR/Cas9 and HDR templates was accomplished by stable gene knockin using MaxCyte electroporation.