Scientific Brief
MaxCyte® Enabled Innovative SARS-CoV-2 Vaccine Development
Abstract
The pandemic caused by SARS-CoV-2 has killed millions worldwide and remains an incredible burden to global health care systems. Although vaccines have proven effective at preventing COVID-19-related mortality, they do not generate sterilizing immunity. Asymptomatic infections can occur in vaccinated populations raising concern over viral spread and new variant emergence. Approved vaccinations are administered intramuscularly and only elicit a systemic immune response. An innovative approach to vaccine design is to target the nasal mucosa and stimulate both systemic and local immunity, thereby reducing infection rates and viral transmission. Here, MaxCyte enabled the development of an intranasal vaccine, AdCOVID, that protected mice from lethal SARS-CoV-2 challenge.1
Experimental Design
The AdCOVID vaccine is a replication-deficient adenovirus type 5 vector that expresses the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. Recombinant plasmids encoding viral genes were electroporated into PER.C6 cells using the MaxCyte STxTM instrument. Virus was purified from cell lysates and vaccine efficacy was tested in mice.
AdCOVID Vaccine Stimulates Immune Response and Protects Against Lethal SARS-CoV-2 Challenge
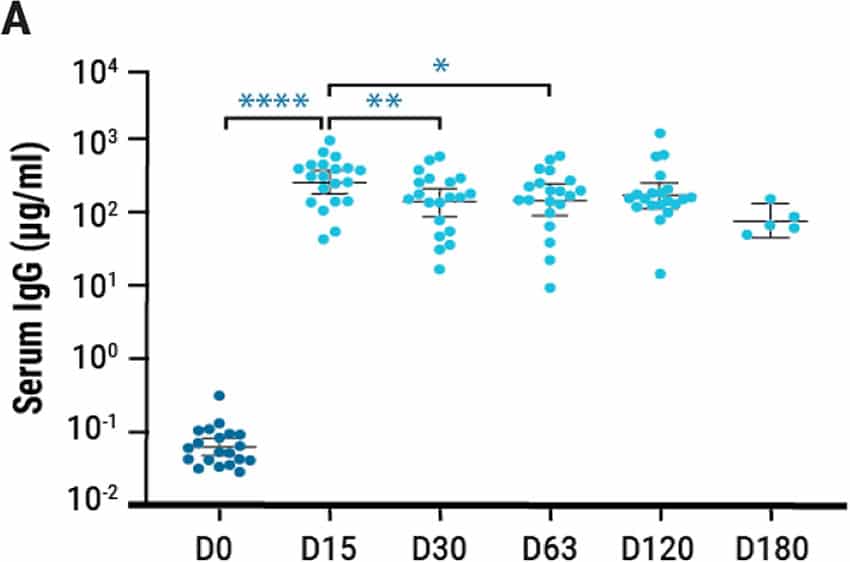
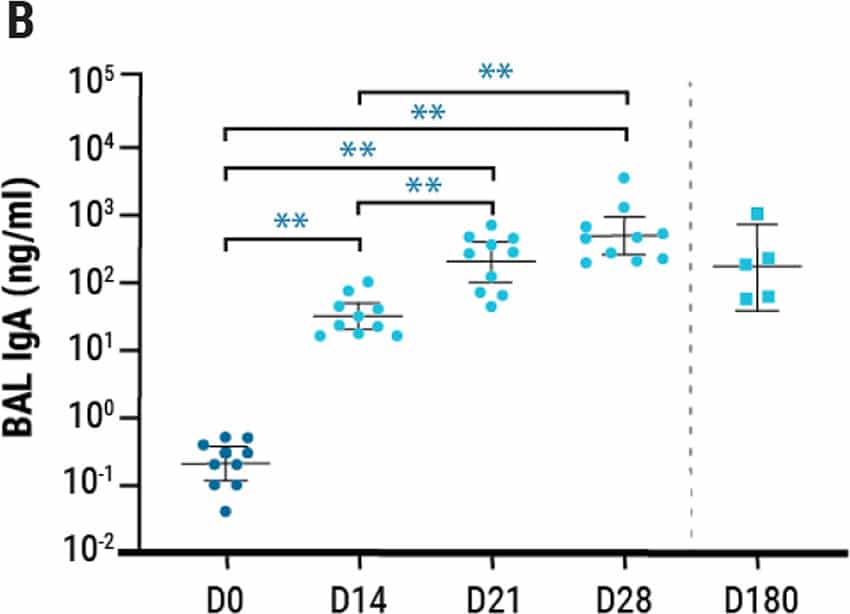
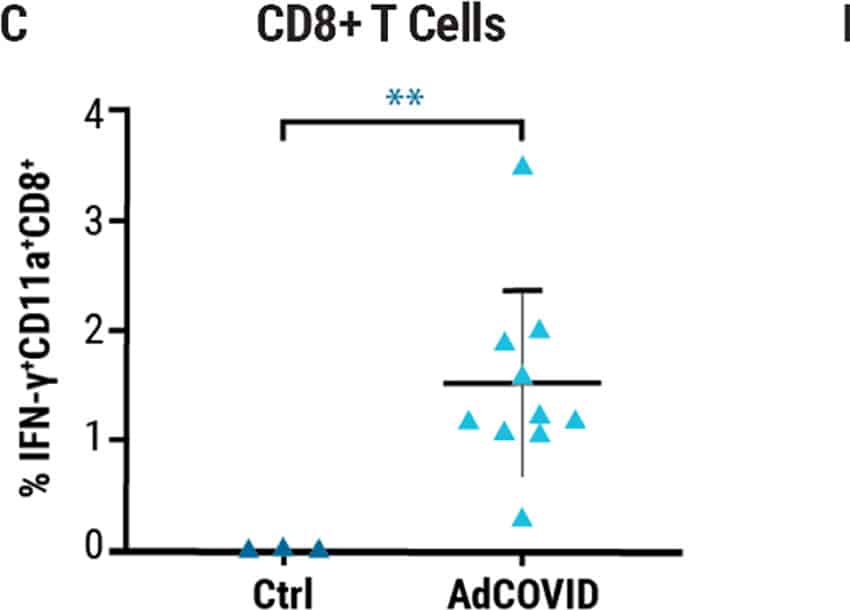
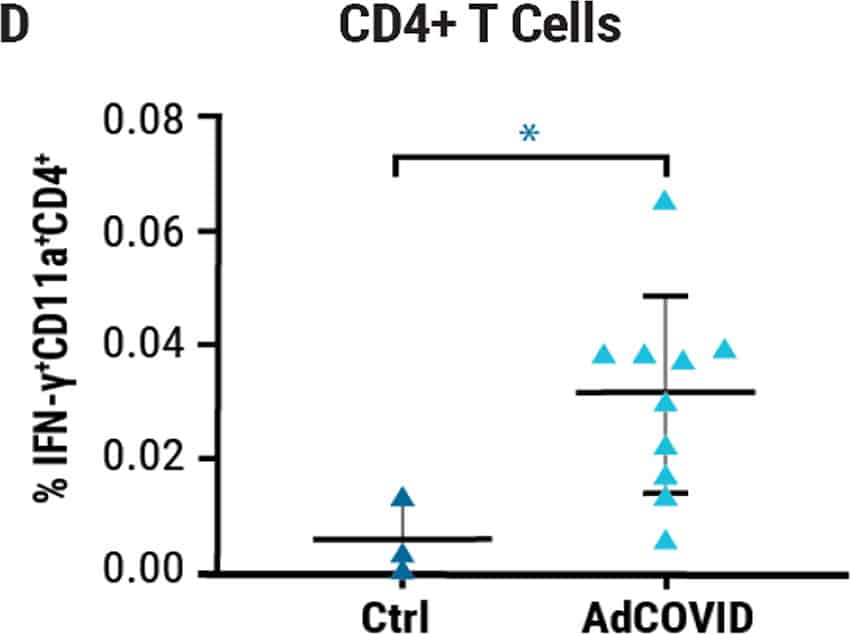
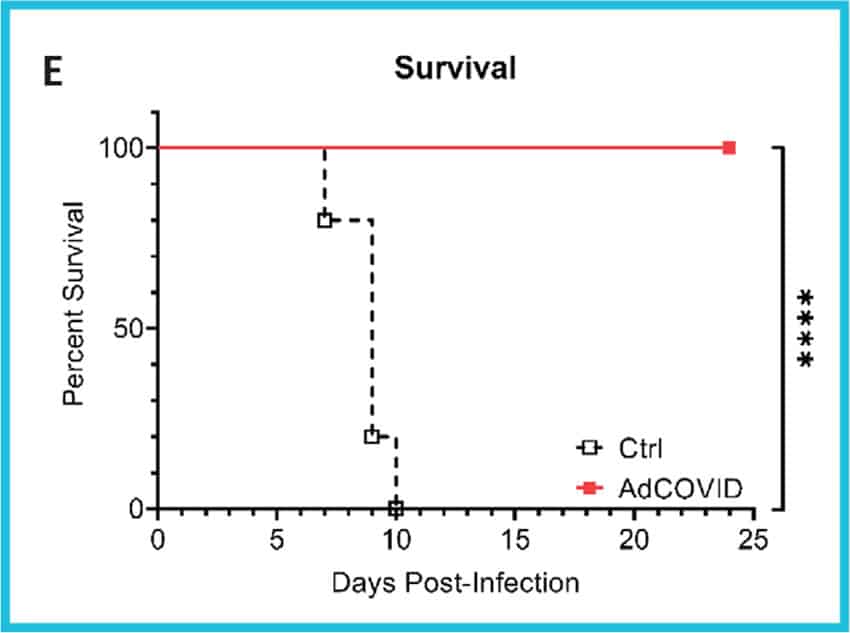
Single intranasal administration of the AdCOVID vaccine resulted in the generation of spike-specific A) IgG antibodies in sera and B) IgA antibodies in bronchoalveolar lavage (BAL) fluids of C57BL/6J mice for at least six months. Lung-localized C) CD8+ and D) CD4+ T cells from vaccinated mice produced interferon-ү (INF-ү) upon restimulation with an RBD peptide pool. E) K18-hACE2 mice were challenged intranasally 28 days post vaccination with the 2019-nCoV/USA-AZ1/2020 strain. All vaccinated mice survived 24 days post infection (DPI), while vehicle control mice succumbed to COVID19- like disease 10 DPI. Vaccination elicited antigen-specific antibody response and polyfunctional T cell activation, both systemically and locally, conferring complete protection against lethal SARS-CoV-2.
Summary
- MaxCyte enabled the modeling of an effective prophylactic AdCOVID vaccine for SARS-CoV-2.
- AdCOVID vaccination completely protected K18-hACE2 mice from lethal SARS-CoV-2 challenge.
- The high efficiency and low toxicity of MaxCyte electroporation enabled preclinical scale vaccine production.
- MaxCyte is an essential biotechnology platform empowering fundamental SARS-CoV-2 research.
References
- King RG, Silva-Sanchez A, Peel JN, et al. Single-Dose Intranasal Administration of AdCOVID Elicits Systemic and Mucosal Immunity against SARS-CoV-2 and Fully Protects Mice from Lethal Challenge. Vaccines (Basel). 2021;9(8):881. Published 2021 Aug 9. doi:10.3390/vaccines9080881
This content was adapted from King et al. 2021 under the Creative Commons license Attribution 4.0 International (CC BY 4.0)





