Webinar: Overview of a Flexible, Clinically Adaptable, Non-viral Approaches to CAR TCR Methodologies
Abstract:
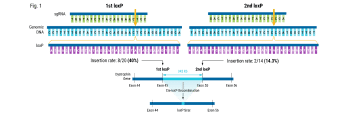
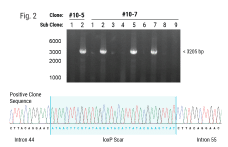
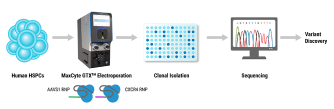
In this webinar for scientists and researchers, Rama Shivakumar, a senior scientist at MaxCyte Inc., highlights powerful case studies that demonstrate the successful use of MaxCyte’s clinically validated, scalable electroporation system in the pre-clinical and clinical scale engineering of resting and activated T cells using a mesothelin specific CARmRNA; in the enhancement of NK cell cytotoxicities against B cell malignancies using an antiCD19 CAR mRNA; in the transposon (Piggybac and Sleeping Beauty) based gene delivery for manufacture of CAR-T cells ; and finally in the gene editing of T cells for improving the efficacy of a TCR immunotherapy.
Watch Presentation

Presenter