The three-day event, organized by president Izuho Hatada, was held at Tower Hall Funabori in Tokyo, and in person for the first time in three years. The event was held as a forum to exchange ideas and thoughts on various topics such as CRISPR-Cas9, new various models used in genome editing and new emerging technologies.
Featured Event
Homology-independent targeted insertion (HITI) enables guided CAR knock-in and efficient clinical scale CAR-T cell manufacturing
Date: June 8, 2023
Time: 12:35 - 1:20 PM (JST)
Presenter: Hyatt Balke-Want
Abstract
Background: Chimeric Antigen Receptor (CAR)-T cell therapies have become an effective therapeutic option for some patients with B cell and plasma cell malignancies and could disrupt the therapeutic landscape of solid tumors. However, access to viral vector engineered CAR-T cell therapies faces significant challenges to meet clinical needs, in large part due to high cost and long lead times for manufacturing GMP vector . Therefore, moving to a non-viral gene delivery platform for the manufacture of CAR-T cell therapies is a logical next step. Non-viral site directed CAR integration can be accomplished using CRISPR/Cas9 and double-stranded DNA (dsDNA) via homology-directed repair (HDR), however yields with this approach have been limiting for clinical application. We discuss here a targeted but HDR-independent approach for the manufacture of CAR-T cell therapies.
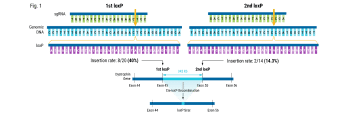
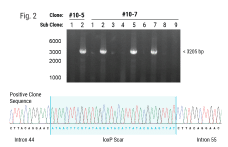
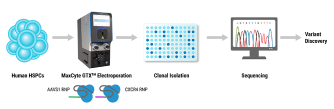
Methods: We applied homology-independent targeted insertion (HITI) or HDR using CRISPR/Cas9 and nanoplasmid DNA to insert an anti-GD2 CAR into the T cell receptor alpha constant (TRAC) locus and compared both targeted insertion strategies. Next, we optimized post-HITI CRISPR EnrichMENT (CEMENT) to seamlessly integrate it into a 14-day process and compared our knock-in with viral transduced anti-GD2 CAR-T cells. Finally, we explored the off-target genomic toxicity of our genomic engineering approach.
Results: Here, we show that site directed CAR integration utilizing nanoplasmid DNA delivered via HITI provides higher cell yield compared to HDR mediated gene insertion. CEMENT enriched CAR T cells to approximately 80% purity, resulting in therapeutically relevant dose ranges of 5.5x108-3.6x109 CAR+ T cells. CRISPR knock-in CAR-T cells were functionally comparable with viral transduced anti-GD2 CAR-T cells and did not show any evidence of off-target genomic toxicity.
Conclusions: Our work provides a novel platform to perform guided CAR insertion into primary human T-cells using nanoplasmid DNA and holds the potential to increase access to CAR-T cell therapies.
Presenter