Scientific Brief
Gene Knockin via Homology-directed Repair in Human iPSCs
Advancing Adoptive Cellular Therapies Using Non-viral-based Engineering
Excitement over recent promising breakthroughs in autologous cellular therapies has been tempered by the manufacturing costs associated with viral-based gene delivery methods and by safety concerns associated with random integration of viral vectors. As an alternative to viral-based gene delivery, we describe the use of the clinically validated and scalable MaxCyte® Flow Electroporation® Technology for delivering ribonucleoprotein (RNP) and single-stranded oligonucleotide donor (ssODN) to induced pluripotent stem cells (iPSCs). Through a restriction fragment length polymorphism (RFLP) assay we show highly efficient gene editing following co-transfection of CRISPR-Cas9 and donor construct.
Case Study
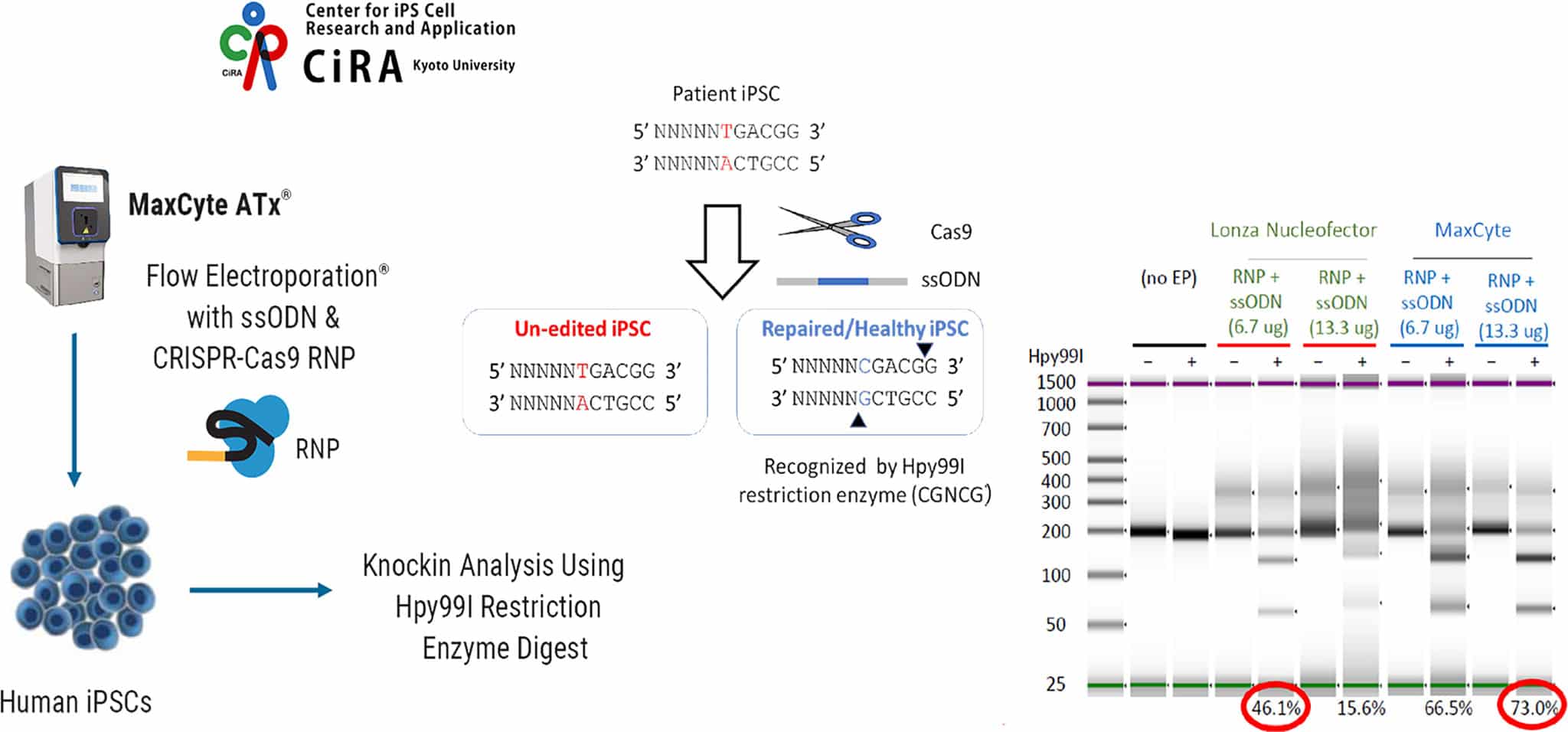
CRISPR-Cas9 Engineering of Patient-derived iPSCs by ssODN Knockin for Efficient and Scarless Genome Editing
In order to generate an isogenic control for drug screening purposes, patient-derived induced pluripotent stem cells (iPSCs) were electroporated by MaxCyte or Lonza Nucleofector® with CRISPR-Cas9 RNP and 100-bp ssODN to repair a premature stop codon from TGA->CGA. iPSCs were harvested three days after electroporation, genomic DNA was extracted, and the genomic region of interest was amplified by PCR. Successful knockin was evaluated by cleavage of the PCR amplicon by Hpy99I restriction enzyme digestion. Data from collaboration with CiRA (U. Kyoto).
Summary
- Non-viral engineering enables rapid development of next-generation therapies with simplified, more cost-effective manufacturing.
- The high efficiency and low toxicity of MaxCyte Flow Electroporation® provides for high knockin frequencies.
- MaxCyte Flow Electroporation Technology (co)delivers a diversity of payloads including mRNA, sgRNA, RNPs, and plasmid & minicircle DNA providing flexibility for sophisticated, non-viral engineering including:
- Transient mRNA expression
- Nuclease-mediated gene editing (CRISPR, TALEN, ZFN)
- Transposon insertion (Sleeping Beauty®, piggyBac®)
- MaxCyte clinical scalability and regulatory compliance provide for streamlined clinical translation of new therapies.





