Article
Innovations in Electroporation Empower Cell- Based Therapeutic Advancement
Introduction
Breakthroughs in genetic engineering have changed how we think about medicine, propelling cell-based therapeutics to the forefront of human health, where previously incurable diseases now have treatment options. The first step in cell engineering is to deliver editing molecules into the cell. Electroporation is a technique that uses electricity to make cell membranes permeable, allowing diverse payloads to enter. Its invention in 1982 by Eberhard Neumann, a biochemist at the Max Planck Institute in Germany, was a revolutionary step forward for genetic engineering. It enabled stable transfections with superior DNA uptake without damaging the cell or its membrane. The technique has since evolved from being performed in a single cuvette to a highly efficient technology for cellular engineering at a commercial scale. Electroporation can be used from development through to manufacturing, expediting therapeutic advancement.
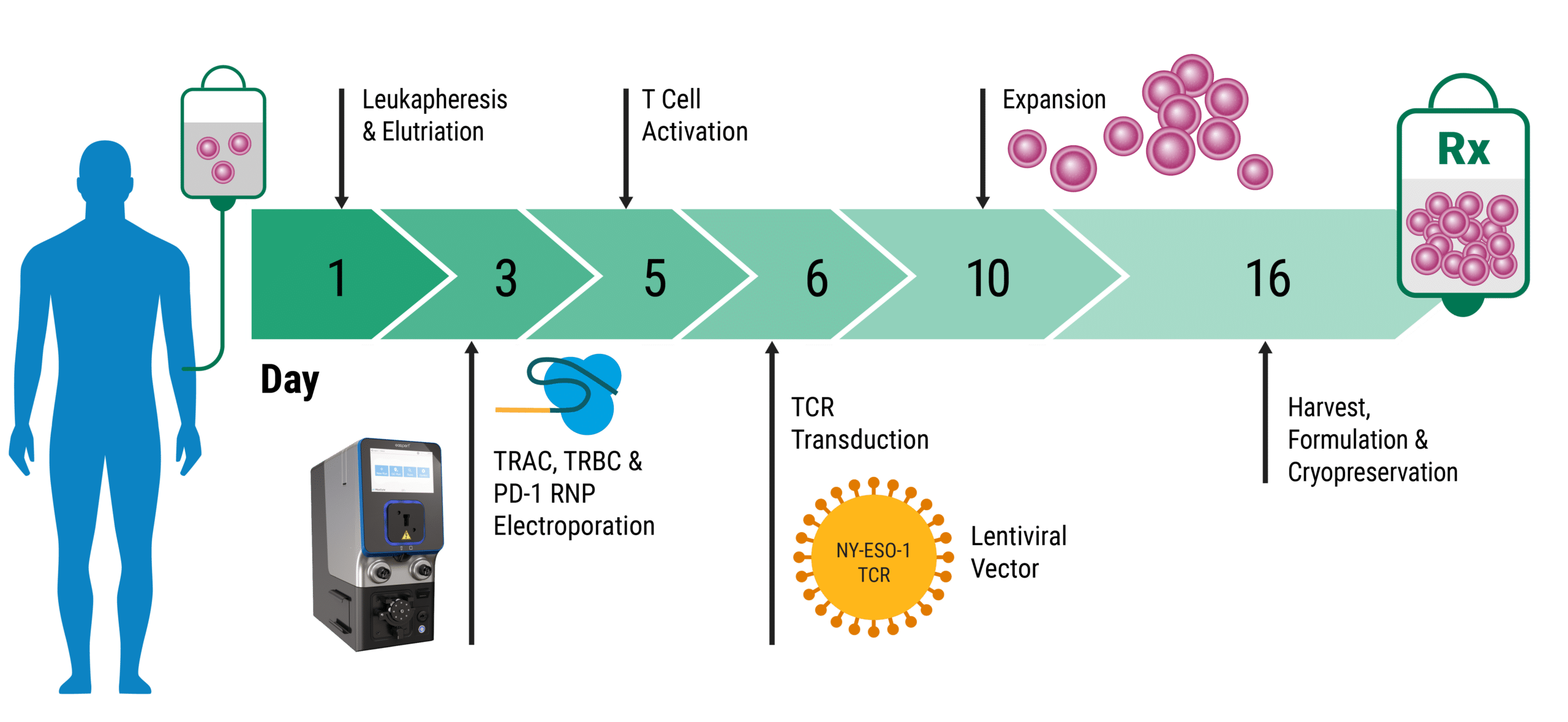
Therapeutic Development with Electroporation
To engineer a cell, molecules that can alter the cell’s genetic code need to cross the plasma membrane. Under normal conditions, many molecules, particularly highly charged ones such as nucleic acids, do not efficiently enter the cell. Several approaches have been developed to facilitate delivery to overcome this limitation, such as using viral vectors and lipid vesicles. Electroporation is a transfection technique where an electric pulse is applied to cells causing transient permeability within the cell membrane. A diverse range of genetic engineering payloads such as DNA, mRNA, CRISPR and Ribonucleoproteins (RNPs) can travel through the membrane and enter the cell. Electroporation is a highly efficient delivery method that can be applied to various cell types, from insect to mammalian, making it an incredibly versatile technique. Whether engineering cells as the next generation of cell or gene therapy, producing protein and antibodies, developing cell-based assays, or driving vaccine development, electroporation facilitates research. CRSIPR-Cas9 technology is one of the most prominent tools for precise gene editing. However, knockin (KI) efficiency and absolute yield of live KI cells remain challenging, particularly in clinical and therapeutic contexts. In a groundbreaking study recently published in Nature Biotechnology, the Marson lab developed a current good manufacturing practice (cGMP) -compliant process to engineer T cells for cancer treatment.1 This team achieved highly efficient knockin of chimeric antigen receptor (CAR) genes into T cells by using electroporation to deliver CRISPR-Cas9 and uniquely designed homology-directed repair (HDR) templates. Highly efficient integration (46-62%) at the notoriously challenging T cell receptor alpha constant (TRAC) locus was achieved with this approach. Electroporation enabled manufacturing of CAR T cells at a clinical scale.
Advances in Electroporation Technology
That have Enabled the Next Generation of Therapeutics
MaxCyte® is transforming the cell engineering landscape, streamlining cell therapy with seamless scalability. This revolution in the field is built on MaxCyte’s pioneering innovation of Flow Electroporation® where cells move through a chamber in a specially designed cuvette called a processing assembly. Discrete volumes of cells are subject to an electric pulse and then collected on a continual basis. With the ability to transfect from 75 thousand up to 20 billion cells on a single instrument, repeated optimization and validation are circumvented. A single complete workflow from concept to commercialization accelerates timelines and reduces costs. In addition, the MaxCyte ExPERT GTx® instrument has an established regulatory path supported by FDA Master File and 21 CFR Part 11 enabled software. These advancements are critical for ensuring rapid, reliable, and safe development pipelines that expedite therapeutics into the clinic, saving patient lives. Efficiently transfecting millions of cells is critical for downstream therapeutics such as engineered T cells. Although CAR T cells are an exciting therapeutic option for treating hematological cancers, a key challenge in the field is their ability to treat refractory cancers. To improve the safety and efficacy of adoptive T cell therapies, researchers have begun to introduce engineered T cell receptors (TCR) as an alternative approach to CARs. In a study conducted by Carl June, published in Science, a bold approach to engineering T cells was investigated for its efficacy in the clinic. MaxCyte electroporation delivered CRISPR RNPs to edit three sites within patient-derived T cells.3 CRISPR RNPs were used to knockout TRAC and TRBC genes to prevent TCR mispairing and improve the expression of cancer-specific transgenic TCRs. In addition, the immune checkpoint inhibitor PD-1 was knocked out to prevent T cell exhaustion. In the first-ever human clinical trial to demonstrate multiplex gene editing in T cells from patients with advanced refractory cancer, the TCR immunotherapy proved durable, safe and effective. Revolutionary innovations such as Flow Electroporation make large-scale genetic engineering strategies a reality, enabling the next generation of cell therapies to reach patients.
Conclusion
Genetic engineering holds the key to breakthroughs in medicine, making engineering strategies pivotal to the feasibility of any cell-based medicine. Although electroporation has been at the heart of genetic engineering for over 40 years, the field has advanced to a place where scalable manufacturing is essential for implementation. Optimization and innovations in foundational techniques, such as electroporation, are necessary for therapeutic advancement. Flow Electroporation offers a solution to some of the toughest challenges in cell and gene therapy such as scalability, cost, timelines, editing efficiency and reproducibility. Clinical trials using this technology highlight how sophisticated CAR and TCR T cell therapies for cancer patients have become a reality. MaxCyte empowers research into therapeutic advancements and enables their transition into the clinic, accelerating the development of cures that will transform human health.
References
- Shy BR, Vykunta VS, Ha A, et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails [published online ahead of print, 2022 Aug 25]. Nat Biotechnol. 2022;10.1038/s41587-022-01418-8. doi:10.1038/s41587-022-01418-8
- Bozza M, De Roia A, Correia MP, et al. A nonviral, nonintegrating DNA nanovector platform for the safe, rapid, and persistent manufacture of recombinant T cells. Sci Adv. 2021;7(16):eabf1333. Published 2021 Apr 14. doi:10.1126/sciadv.abf1333
- Stadtmauer EA, Fraietta JA, Davis MM, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367(6481):eaba7365. doi:10.1126/science.aba7365





