Scientific Brief
MaxCyte® Enables Rapid, High-Yielding, Stable Cell Line Development
Abstract
MaxCyte’s highly efficient Flow Electroporation® has extended the potential use of transient protein production in CHO cells as a tool for biotherapeutic development. However, stable monoclonal CHO cell lines remain the system of choice for manufacturing clinical-grade biologics. Stable cell line development continues to be a bottleneck; a time-consuming, costly and labor-intensive process, often requiring specialized equipment. Here, we present a method for generating high-yielding stable CHO clones within six weeks of transfection.
Experimental Design
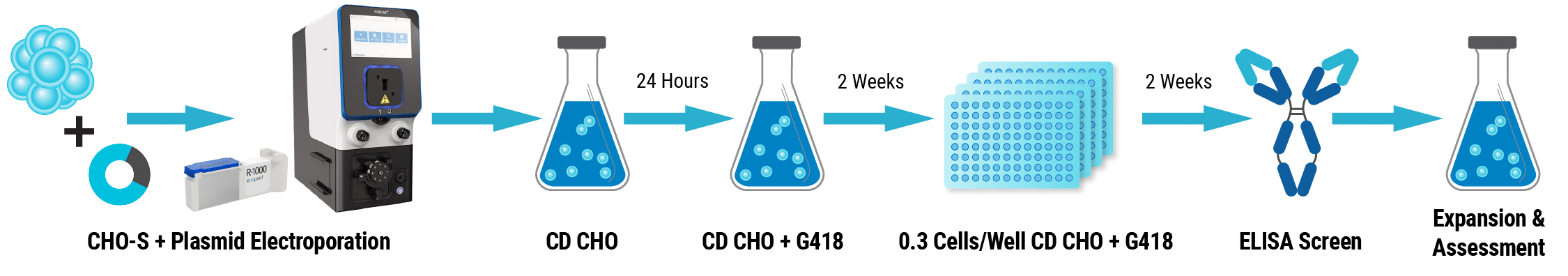
Electroporation
An IgG expression plasmid was added (1-2 μg DNA/106 cells) to CHO-S cells in MaxCyte electroporation buffer (2x108 cells/mL). Cells were transferred to a processing assembly and electroporated on the MaxCyte STXTM using the pre-loaded CHO program.
Culture and Selection
Cells rested for 30 minutes and were then seeded (4x106 cells/mL) in CD CHO (24 hours at 37°C). Cells were harvested via centrifugation and cultured in CD CHO with G418 for two weeks.
Limiting Dilution and Screening
Cells were seeded (0.3 cells/well) into 25 x 96-well plates in CD CHO with G418 for two weeks. 479 clones were screened by ELISA. The 23 top-producing candidates were expanded in shake flasks. Productivity was assessed up to day 21 of culture.
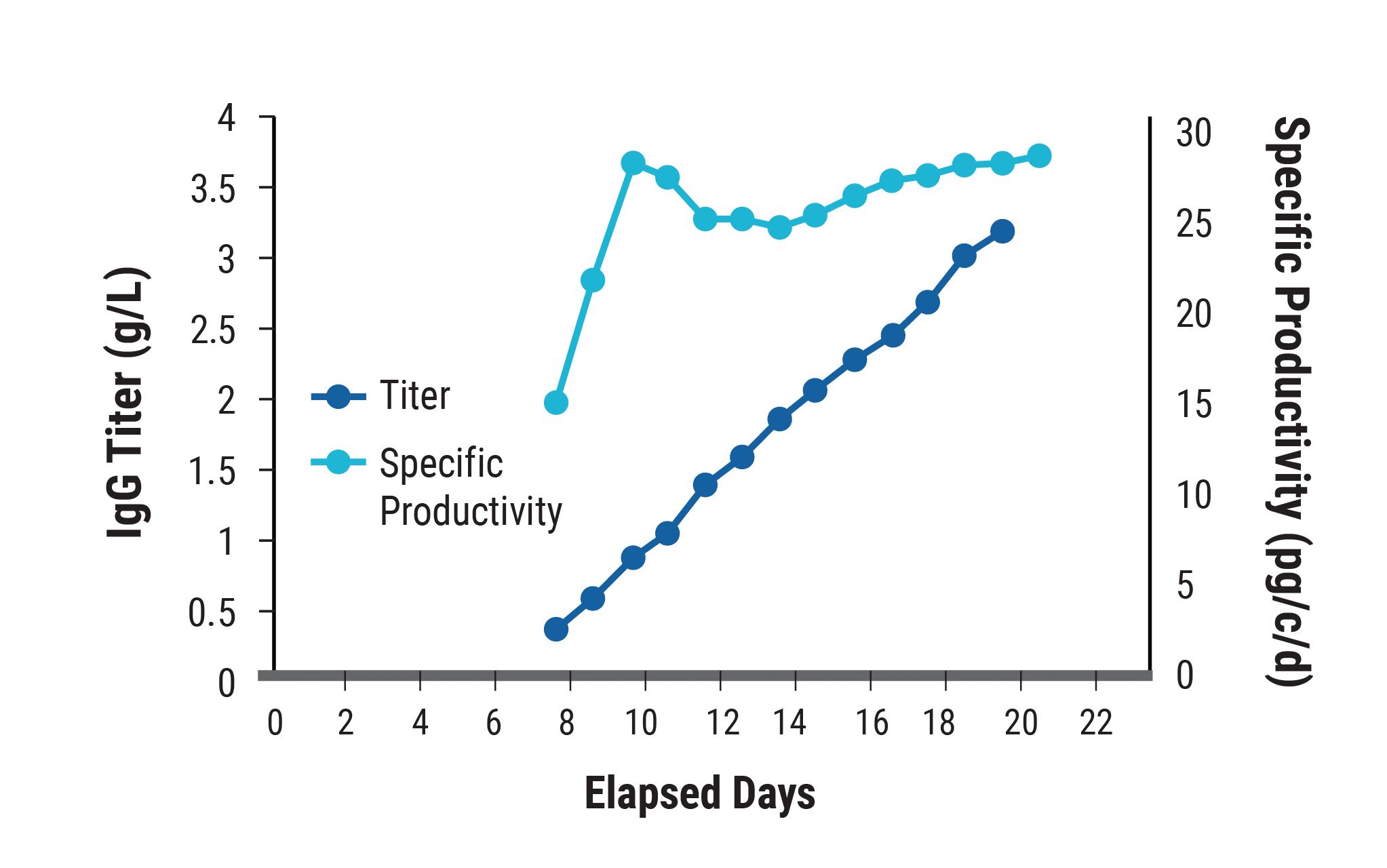
A)
B)
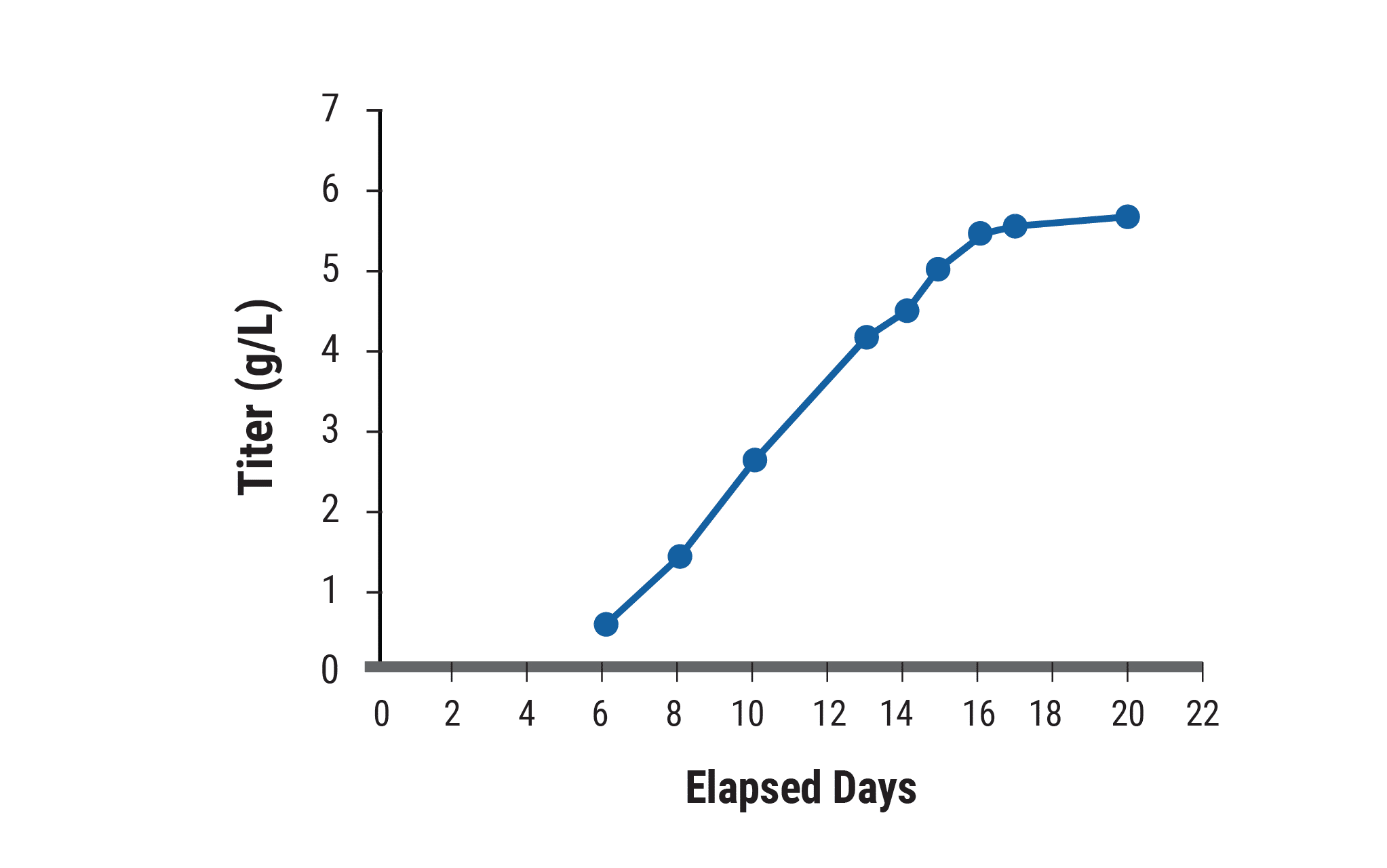
The top-performing clone, identified within six weeks, was examined for yield and productivity. A) In unoptimized culture conditions, specific productivity reached 27 pg/cell/day and remained high over the 21-day production period, when the final antibody titer reached 3.4 g/L. B) With process development, including optimized media/feed strategy, titers exceeded 5.7 g/L.
Summary
- MaxCyte enables rapid, simple, cost-effective CHO-S stable cell line development within six weeks of electroporation.
- High transfection efficiency and cell viability eliminate the need to screen thousands of candidate clones.
- Lower consumable costs
- Less hands-on time - Simplified development with limiting dilution cloning directly from a stable bulk pool.
- Avoid multiple rounds of subcloning and screening
- No need for specialized equipment - Large-scale electroporation of up to 2x1011 cells enables multigram transient protein expression in parallel with stable cell line development.
Steger K, Brady J, Wang W, Duskin M, Donato K, Peshwa M. CHO-S antibody titers >1 gram/liter using flow electroporation-mediated transient gene expression followed by rapid migration to high-yield stable cell lines.
J Biomol Screen. 2015 Apr; 20(4):545-51. Copyright (2015) with permission from Elsevier.
* Since the publication of this study, the new ExPERT STx® instrument has been released to include enhanced software, an improved user interface and an integrated touch screen.





