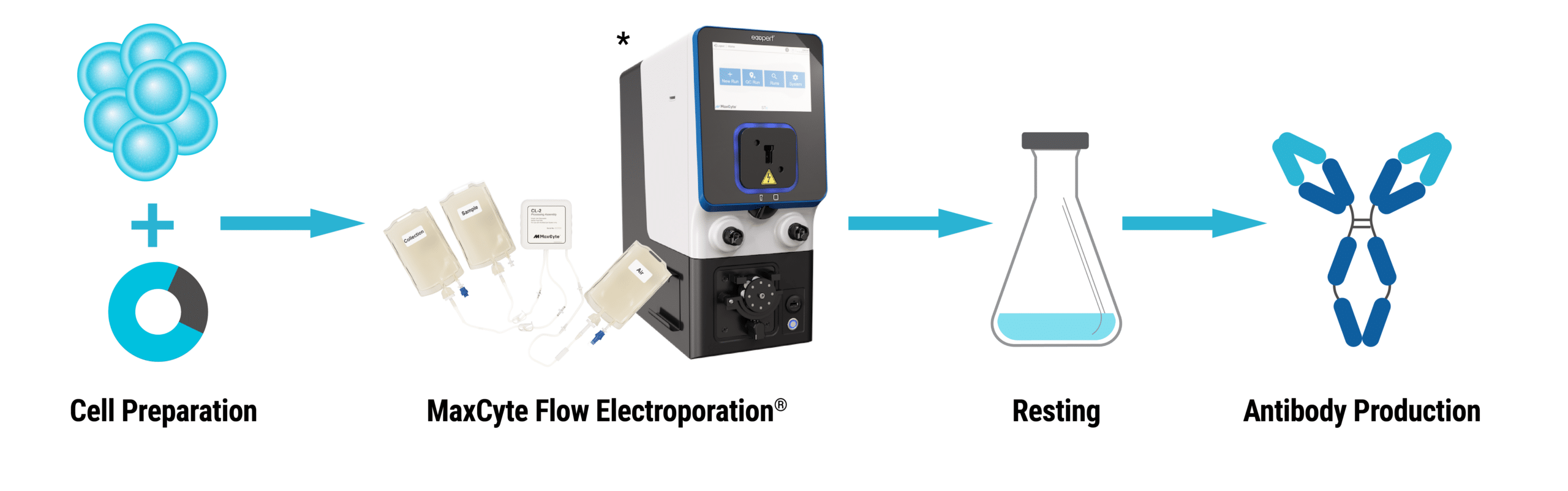
Application Note
MaxCyte® Flow Electroporation® for Gram-Scale Transient Antibody Production in CHO-S Cells
Background
Stable CHO cell lines remain the system of choice for clinical-grade biotherapeutic manufacturing. However, during early-stage development, hundreds of candidates may be under evaluation; creating stable cell lines for each would require prohibitive investments of time and money. As a result, the industry has looked to transient gene expression (TGE) to rapidly screen large numbers of antibodies or antibody-like molecules to identify promising leads for further evaluation.
Poor transfection efficiency, cell viability and yields limited the use of CHO-based TGE, leading the industry to favor HEK-based transient systems. Differences in the manufacturability, affinity and efficacy of antibodies produced in HEK cells compared to those produced by stable CHO cells increase the risks associated with biotherapeutic development.1 Thus, there is great interest in developing CHO-based TGE systems that can rapidly express grams of antibodies, eliminating the need to use HEK-based expression systems.
Various CHO TGE methods are available, including systems based on engineered CHO cells, unique expression constructs and specialized reagents. These systems have varying reproducibility, scalability and cost-effectiveness and generally produce low antibody titers for typical IgG molecules and even lower yields of difficult-to-express molecules such as bispecific antibodies.2
A transient CHO system that easily scales from milligram to multi-gram antibody production would extend the potential of CHO TGE as a tool for both early and mid-stage biotherapeutic development.
MaxCyte® created Flow Electroporation® to enable the development and manufacture of engineered cell therapies. Flow Electroporation delivers superior transfection efficiency and cell viability to various cell types with a range of loading agents, and can transfect up to 2x1011 cells in one reaction. This fully scalable technology enables high-titer antibody production from CHO-based TGE without requiring specific expression constructs, adapted CHO lines, specialized reagents or media additives.
Aim
Here, we present a method for scalable, high-titer antibody production by TGE in CHO-S cells following MaxCyte electroporation, and discuss parameters which can be optimized to maximize yields.2
Optimized Method Overview
Cell culture – Suspension-adapted CHO-S cells were split one day before electroporation.
Cell preparation – Cells were pelleted (250 xg for 10 minutes), resuspended in MaxCyte Electroporation Buffer (2x108 cells/mL), and plasmid DNA was added.
Electroporation – The reaction mix was transferred to a Processing Assembly, loaded onto the MaxCyte STX™ and electroporated using the pre-loaded CHO protocol.
Resting – Cells were immediately transferred to shake flasks and incubated for 30 to 40 minutes (37°C, 5% CO2).
Antibody production – Cells were seeded (6x106 cells/mL) in shake flasks in supplemented CD OptiCHO™ medium. At 24 hours post electroporation (hpe), sodium butyrate (1 mM) was added and the temperature was lowered to 32°C. Cells were fed periodically with a commercially available supplement.
Results
MaxCyte Electroporation Enabled High-Efficiency, High-Viability CHO-S Transfection
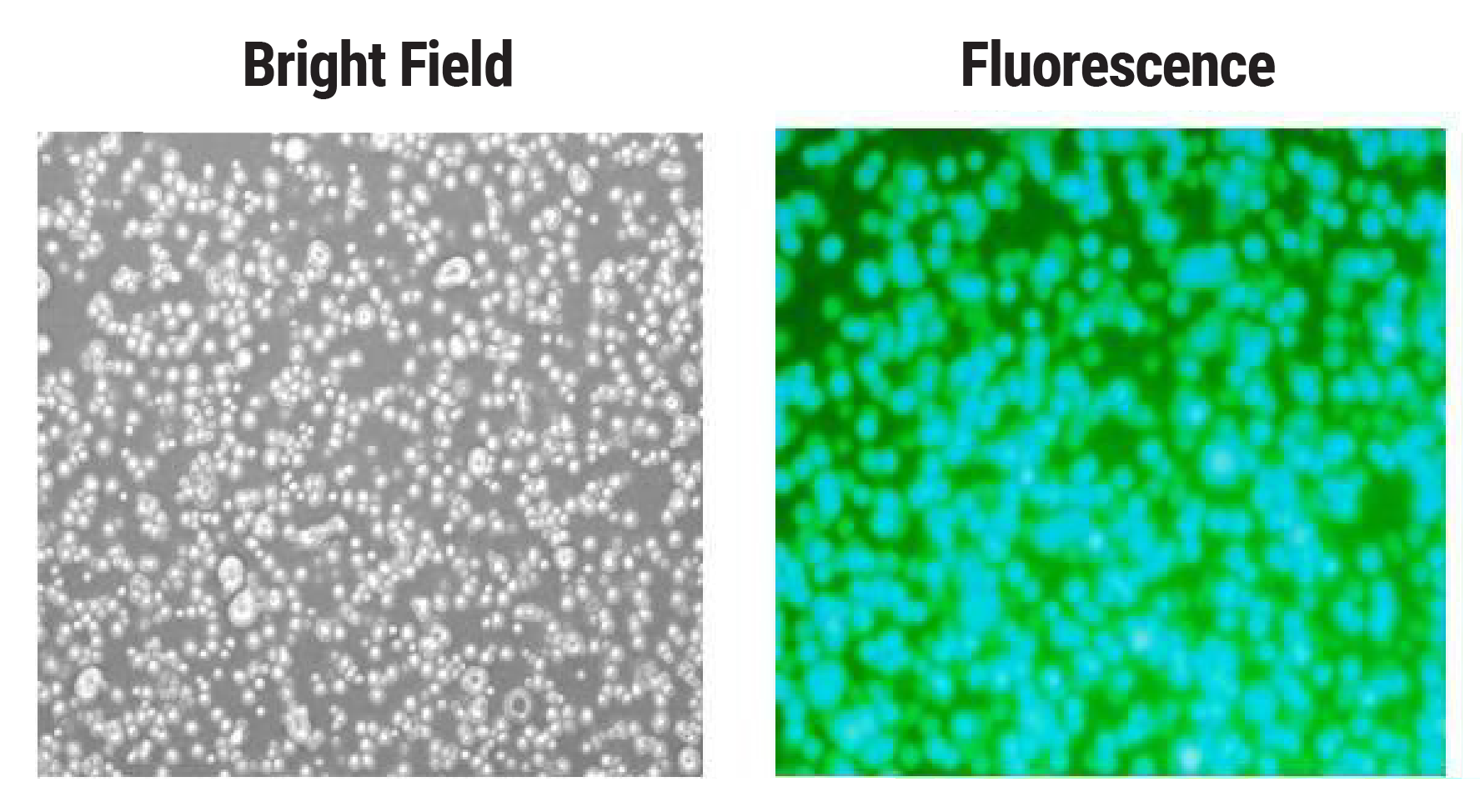
Transient transfection of CHO cells has historically resulted in lower transfection efficiencies than other cell types used for protein expression, such as HEK cells. To assess transfection efficiency and cell viability following MaxCyte electroporation, CHO-S cells were transfected with a plasmid encoding a green fluorescent protein. GFP expression measured at 24 hpe shows over 95% transfection efficiency (Figure 1).
A)
B)
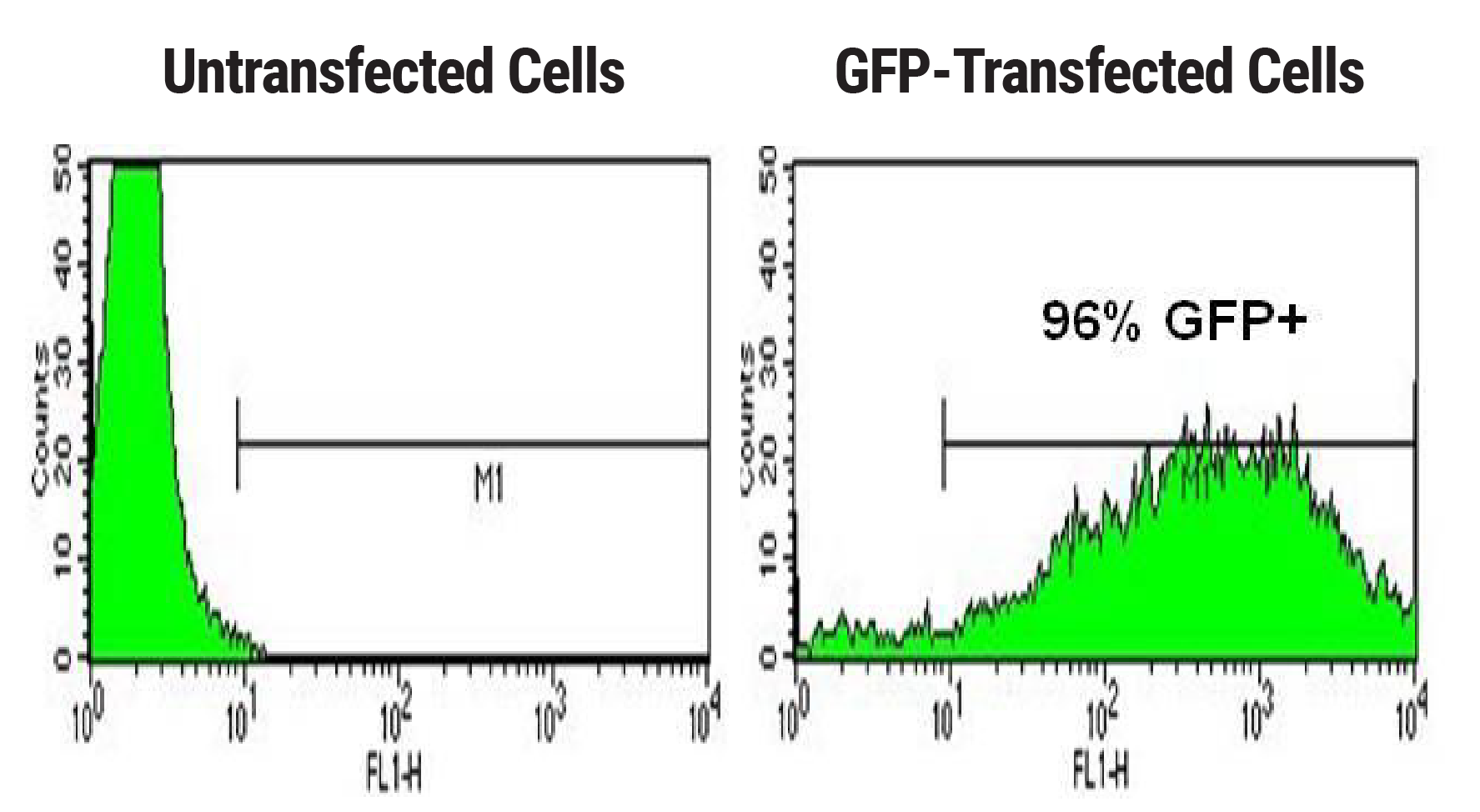
Figure 1. High transfection efficiency following CHO cell electroporation. CHO-S cells were electroporated with a plasmid encoding GFP. Cell viability (data not shown) and GFP expression were measured 24 hours post electroporation via A) microscopy and B) flow cytometry.
Strategies for Increasing Yield and Laboratory Productivity
Various cell culture parameters that affect productivity have been identified, including cell seeding density, feeding conditions, media additives and culture temperature. The researchers used small-scale electroporation experiments to identify conditions that yielded the highest antibody titers.
Optimizing Media and Feed Strategies Improves Yields and Reduces Costs
Cell growth, nutrient depletion and protein yields are interrelated and influenced by media composition and culture conditions. To optimize these variables, electroporated CHO-S cells were cultured using different strategies. The combination of a hypothermic shift and the addition of sodium butyrate increased yields over 6-fold compared with basal conditions (Figure 2). Optimizing the feed strategy produced further gains, enabling titers to exceed 1.2 g/L within two weeks (Figure 3).
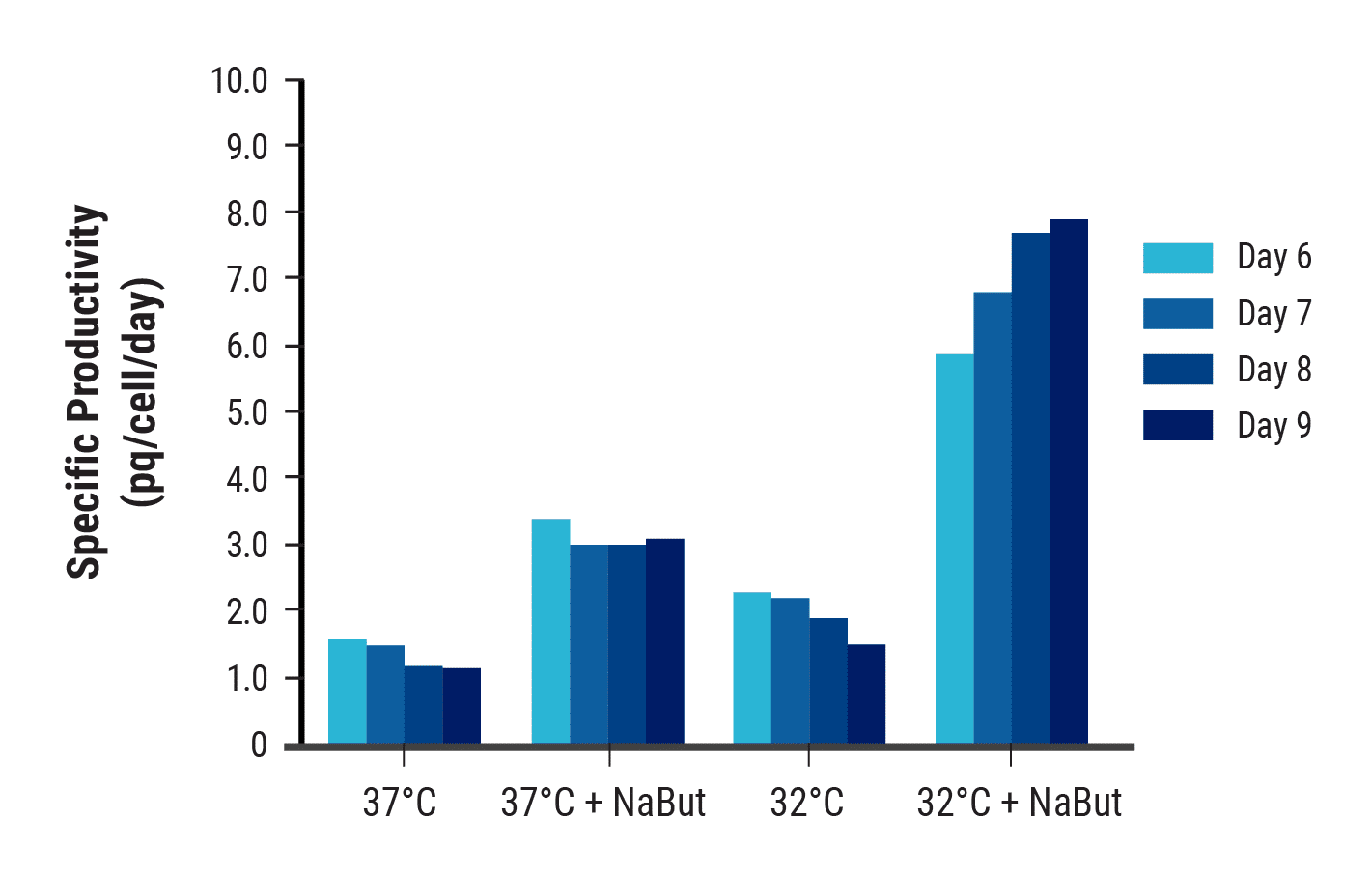
Figure 2. Temperature shift and the addition of sodium butyrate increased antibody production. CHO-S cells were electroporated with an antibody expression plasmid. Post electroporation, cells were cultured in CD CHO medium. At 24 hpe, the temperature was shifted to 32°C and 1mM sodium butyrate (NaBut) was added as indicated. Antibody titers were measured by ELISA and the specific productivity was calculated.
B)
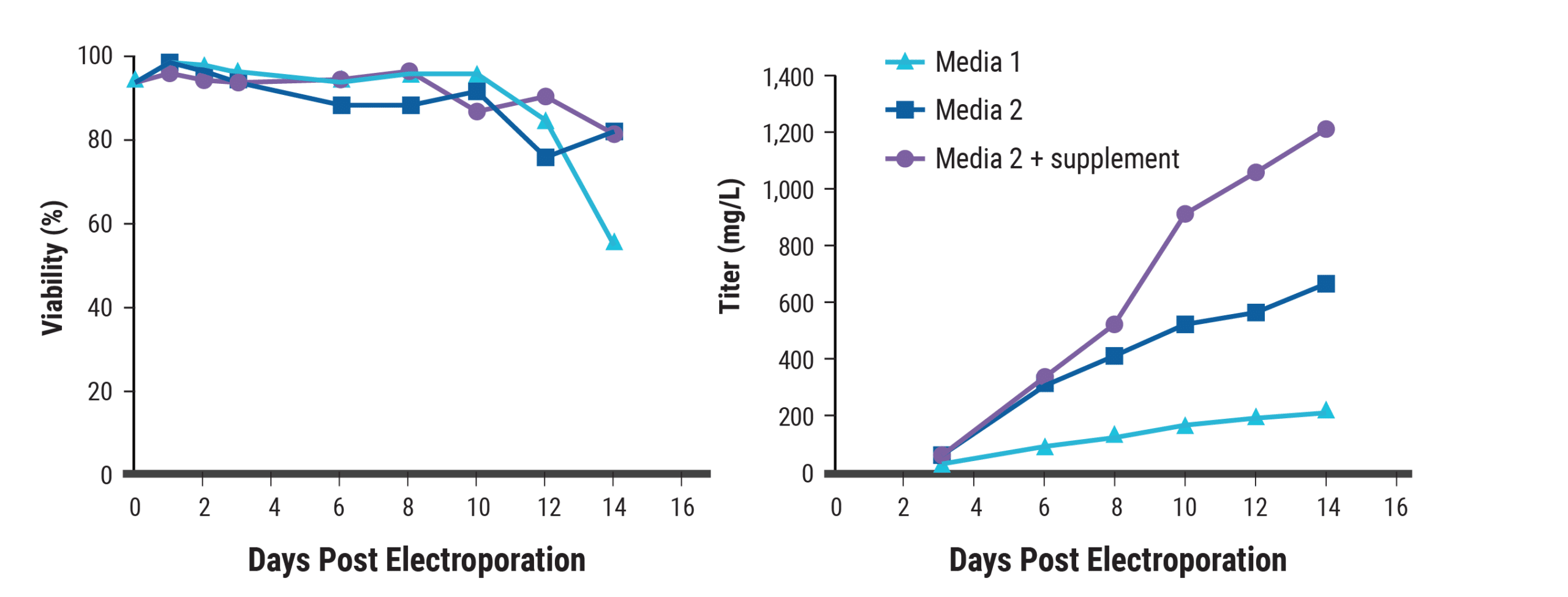
Figure 3. Optimizing media yields higher antibody titers. Using small-scale electroporation, CHO-S cells were electroporated with an antibody expression plasmid. Post electroporation, cells were seeded at 5 to 6×106 cells/mL in supplemented or unsupplemented CD CHO or CD OptiCHO media. At 24 hpe, sodium butyrate (1 mM) was added and the temperature was lowered to 32°C. Cells were fed periodically. A) Cell viability and B) antibody titers were measured.
Optimizing Cell Culture Seeding Density Improves Productivity
Higher cell densities in intensified cultures minimize media usage and can simplify downstream processing, improving productivity. Using unoptimized feed conditions, antibody yields from transfected CHO cells at medium (6x106 cells/mL) and high (1x107 cells/ml) cell seeding were analyzed. High-density cultures produced over 2.5-fold greater antibody titers than medium-density cultures, exceeding 800 mg/L within 15 days of transfection (Figure 4).
A)
B)
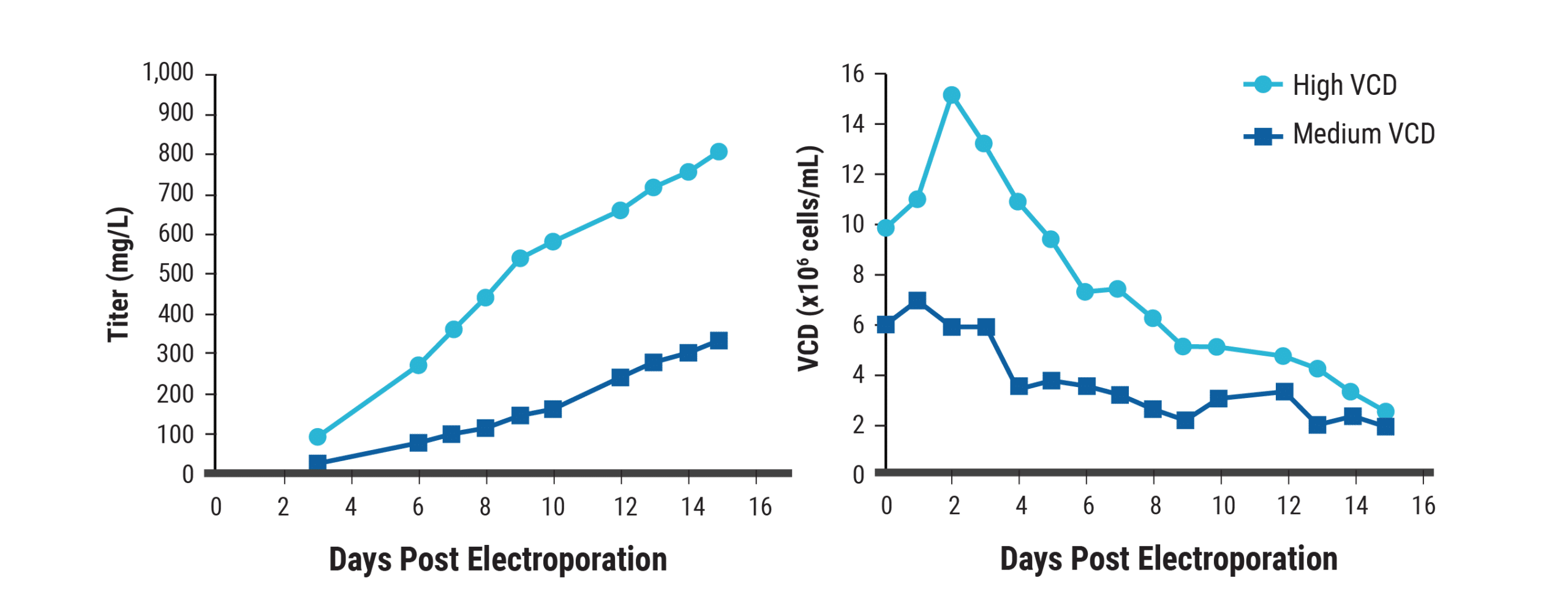
Figure 4. Increased antibody titers upon culturing at high cell densities. CHO-S cells (1x1010 cells) were electroporated with an antibody expression plasmid. Electroporated cells were inoculated into shake flasks at high (1x107 cells/mL) or medium (6x106 cells/mL) seeding densities. Cells were cultured in CD OptiCHO medium and fed periodically with commercially available supplements. At 24 hpe, sodium butyrate (1 mM) was added and the temperature was lowered to 32°C. A) Antibody titers and B) viable cell densities were measured.
Electroporation and Culture Conditions are Scalable
Using small-scale electroporation to optimize conditions for TGE is convenient and cost-effective. However, it is essential that those conditions can be applied to large-scale electroporation without further optimization. To demonstrate the scalability of MaxCyte electroporation and selected culture conditions, CHO-S cells were transfected by either small-scale (8x107 cells) or large-scale (1x1010 cells) electroporation, then batch cultured in 20 mL (small-scale) or 2.8 L (large-scale) following the optimized method described above.
Both cultures yielded titers exceeding 1 g/L; the small-scale reaction produced 21 mg of protein, and the large-scale reaction produced 3.5 g by day 12 (Figure 5). MaxCyte electroporation enabled the rapid identification of optimal transfection and culture conditions which can be seamlessly scaled for large-scale antibody production.
A)
B)
C)
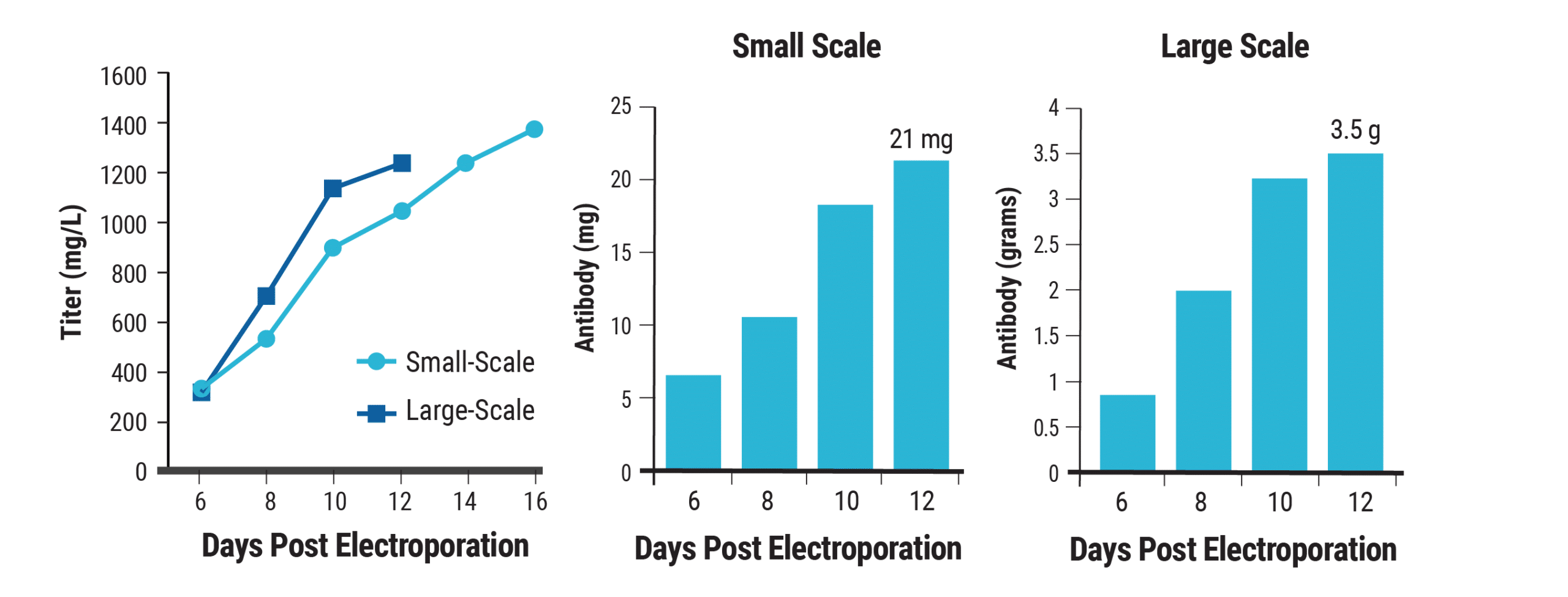
Figure 5. MaxCyte electroporation scales seamlessly for gram-scale antibody production. CHO-S cells were transfected with an antibody expression plasmid at small (8×107 cells) or large-scale (1×1010 cells). At 24 hpe, sodium butyrate (1 mM) was added the temperature was lowered to 32°C. Cultures were fed periodically. A) Antibody titers were measured on the days shown. Total antibody yields following electroporation at B) small-scale and C) large-scale were calculated.
Conclusion
This study used small-scale electroporation to identify optimal conditions that could be applied to large-scale transfection without loss of productivity or the need for further optimization. While the chosen parameters increased productivity for this combination of target protein and cells, MaxCyte technology enables researchers to rapidly optimize transfection conditions in their preferred eukaryotic expression system and cells of interest.
The high productivity of MaxCyte-driven CHO TGE allows for its use in early-phase candidate identification and for generating the antibody quantities needed for later-stage pharmacology, stability and manufacturability studies. Using CHO in early development instead of HEK can reduce developmental delays by removing the need to reoptimize conditions and revalidate results when switching cell lines.
MaxCyte electroporation facilitates the identification of quality candidates and their rapid progression through pre-clinical studies by enabling efficient TGE in CHO cells at any scale.
References
- Croset, A.; Delafosse, L.; Gaudry, J. P.; et al. Differences in the Glycosylation of Recombinant Proteins Expressed in HEK and CHO cells. J. Biotechnol. 2012, 161, 336–348. doi: 10.1016/j.jbiotec.2012.06.038
- Steger, K., Brady, J., Wang, W., et al. CHO-S Antibody Titers >1 Gram/Liter Using Flow Electroporation-Mediated Transient Gene Expression followed by Rapid Migration to High-Yield Stable Cell Lines. J Biomol Screen. 2015 Apr;20(4), 545-551. doi: 10.1177/1087057114563494
* Since the publication of this study, the new ExPERT STx® instrument has been released to include enhanced software, an improved user interface and an integrated touch screen.
Reprinted from J Biomol Screen, 20(4), Krista Steger, James Brady, Weili Wang, Meg Duskin, Karen Donato, Madhusudan Peshwa. CHO-S Antibody Titers >1 Gram/Liter Using Flow Electroporation-Mediated Transient Gene Expression followed by Rapid Migration to High-Yield Stable Cell Lines. 545-551., Copyright (2015) with permission from Elsevier.





