Scientific Poster
Non-Viral Engineering of Human iPSCs to Manufacture Rejuvenated TCR T Cells for Cancer Immunotherapy
Abstract
Engineered, human induced pluripotent stem cells (iPSCs) offer a replenishable source to differentiate large numbers of T cells for cancer immunotherapy applications. However, the transduction efficiency of iPSCs by retroviral and lentiviral vectors is suboptimal, and there are safety concerns owing to the presence of viral gene products. Therefore, we explored using a non-viral, cGMP compliant, electroporation platform to transfect TCR-expressing piggyBac® transposons into human iPSCs. Three promoters were evaluated for optimal expression of a surface marker, and iPSCs were analyzed 14 days after electroporation by flow cytometry to determine the transposition efficiency. Remarkably, up to ~47% of cells expressed the surface marker protein without selection. Subsequently, iPSCs were electroporated to compare several TCR molecules. TCR-expressing iPSCs were differentiated into T cells and then evaluated for cytotoxic activity when co-cultured with peptide-pulsed target cells. Potent antigen-specific cytotoxicity was observed. Finally, iPSC-derived T cells expressing several TCR candidates were injected multiple times into mice engrafted with a luciferase-expressing liver cancer cell line. Significant tumor reduction was observed by Day 23 after the initial tumor cell introduction. These results demonstrate efficient TCR DNA transposition into iPSCs, followed by differentiation into T cells with functional activity both in vitro and in vivo.
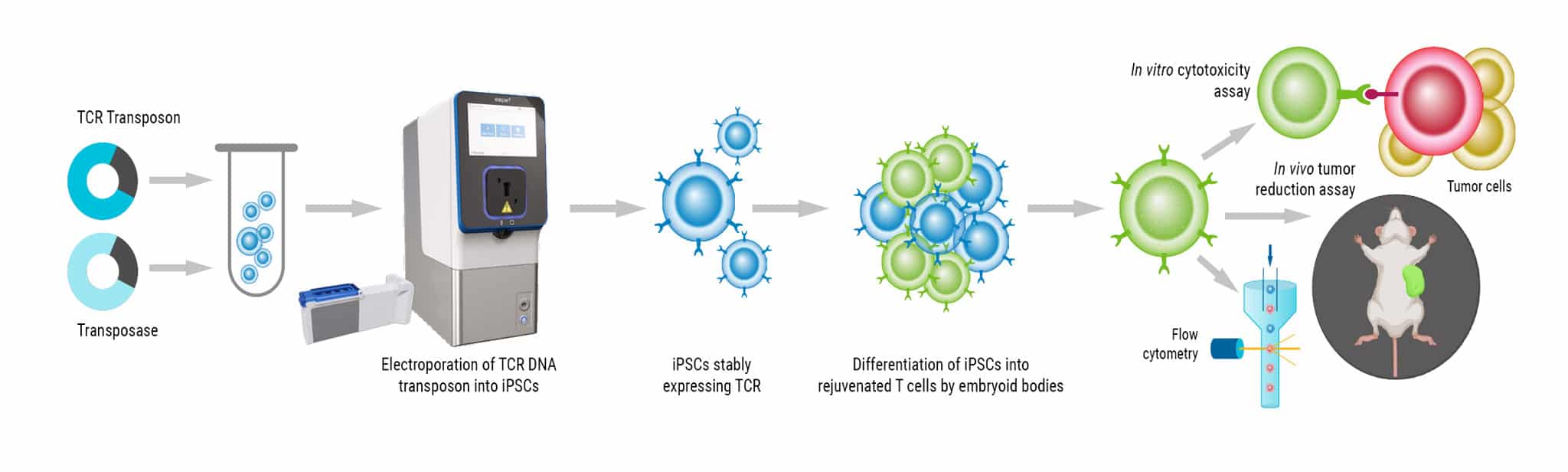
DNA Transposon Electroporation into Human iPSCs and Differentiation into Rejuvenated T Cells
Transgene Expression in iPSCs

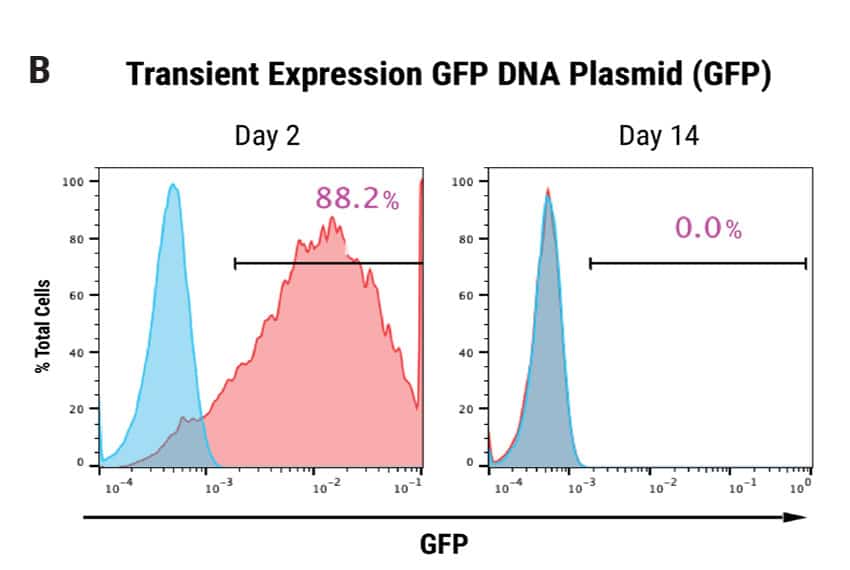
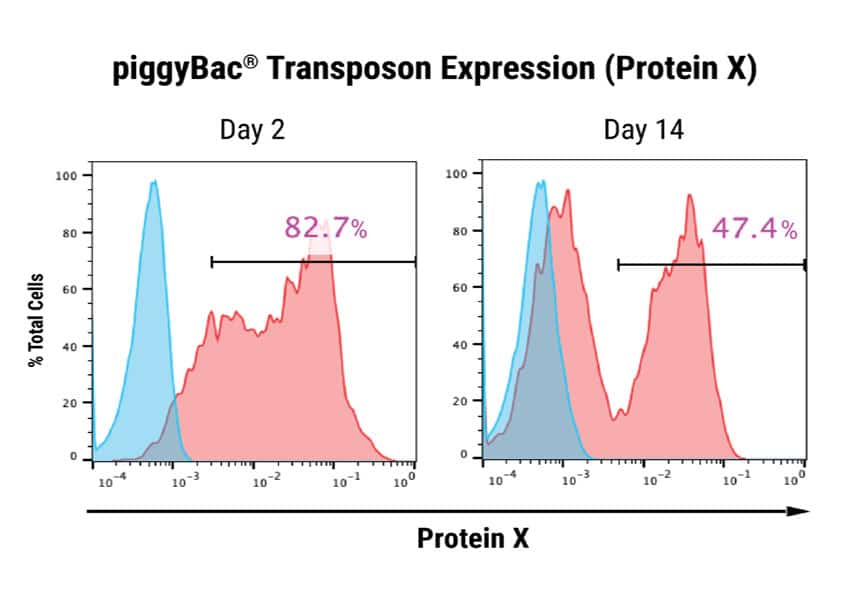
Figure 1: Optimization of plasmid delivery to human iPSCs and stable integration of a DNA transposon. A) Human iPSCs were electroporated with 3 different plasmids expressing piggyBac transposon and a surface marker Protein X, driven by either the CMV promoter, promoter #1 or promoter #2. A separate DNA plasmid expressing the piggyBac transposase was co-transfected. A ratio of either 1:1 or 1:3, transposon:transposase was evaluated. Cells were analyzed by flow cytometry 48 hours post electroporation. Promoter #2 provided optimal expression (320 μg/mL DNA concentration, 1:1 DNA ratio.) B) Human iPSCs transfected with either GFP or piggyBac transposon and transposase (320 μg/mL, 1:1 ratio.) Cells were analyzed 2 days or 14 days post electroporation for expression of GFP or Protein X. GFP expression was detected in 88.2% of total cells, and was undetectable after 14 days. Protein X expression was 82.7% after 48 hours and persisted at 47.4% up to 14 days following electroporation, indicating stable integration of the transgene.
Potent Cytolytic Activity of Rejuvenated T Cells
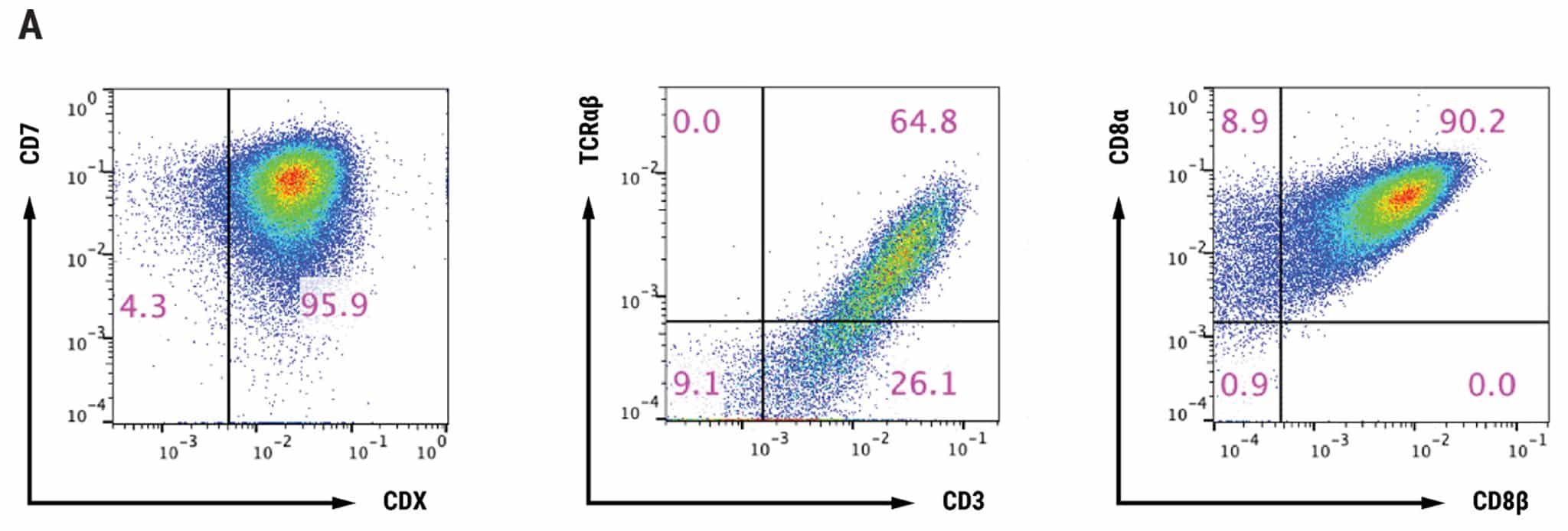
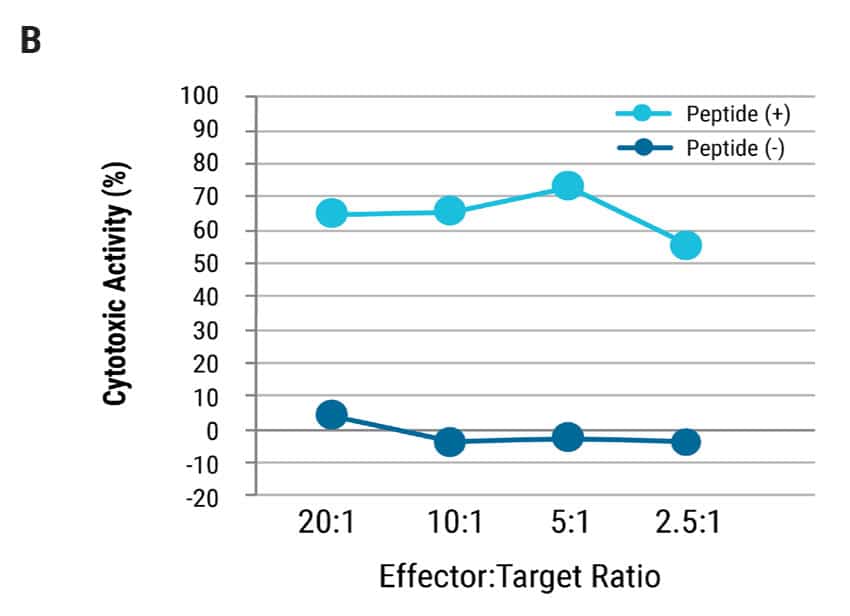
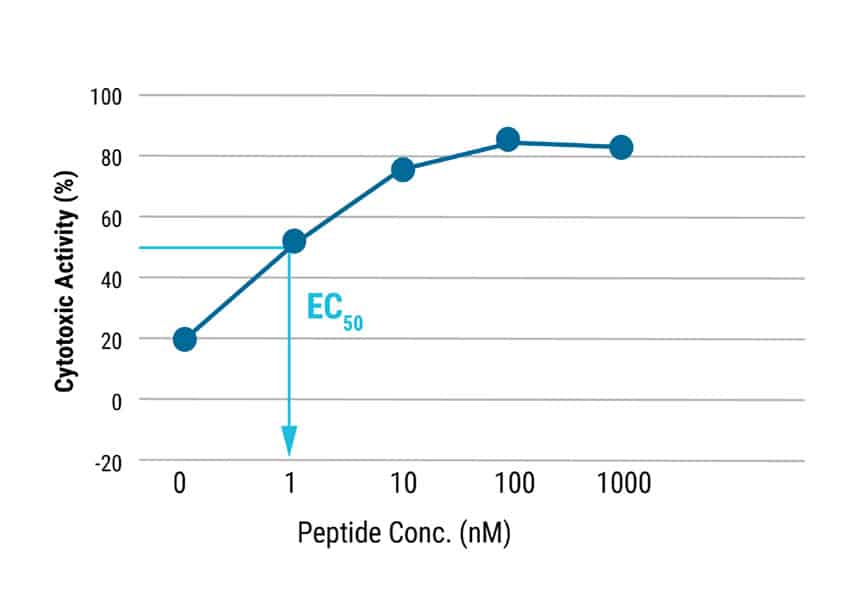
Figure 2: T cell characterization and cytotoxic activity. A) T cells differentiated from iPSCs, were examined for CD7, Protein X, TCR, CD3, CD8α and CD8β expression by flow cytometry. B) TCR T cells were co-cultured with antigen presenting cells pulsed with/without antigen peptide at various effector:target ratios and analyzed for cytotoxic activity. Even at the lowest effector:target ratio of 2.5:1, over 50% cytolytic activity was observed. The EC50 was determined to be approximately 1 nM of target peptide.
Potent Cytolytic Activity of Rejuvenated T Cells
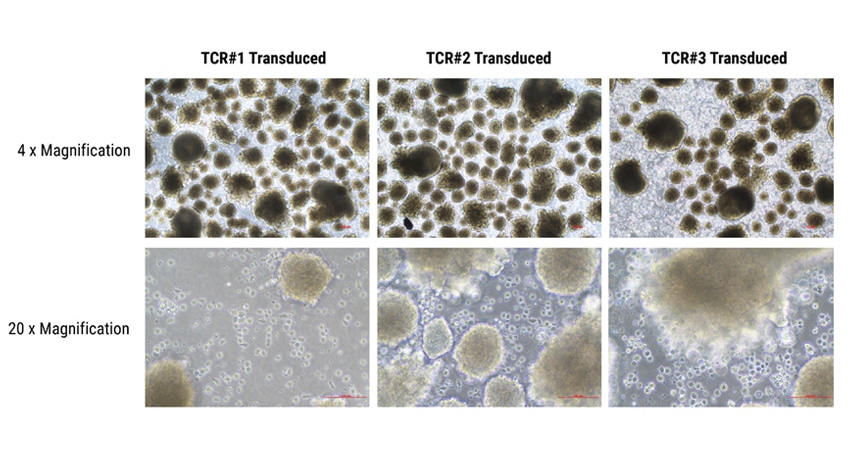
Figure 3: Differentiation of T cells from embryoid bodies. Human iPSCs were electroporated with three TCR piggyBac DNA transposon constructs (TCR #1, TCR#2, or TCR#3). Stable TCR expressing cells were were dissociated into single cells for generation of embryoid bodies, using Ultra-Low Attachment plates under feeder- and serum-free conditions. The addition of several hematopoiesis-specific cytokines resulted in the production of hematopoietic cells which were then differentiated into CD8αβ T cells and matured. Aggregates in the image are embryoid bodies; cells surrounding the embryoid bodies are hematopoietic progenitor cells.
T Cell Suppression of Tumor Development in an in vivo Model
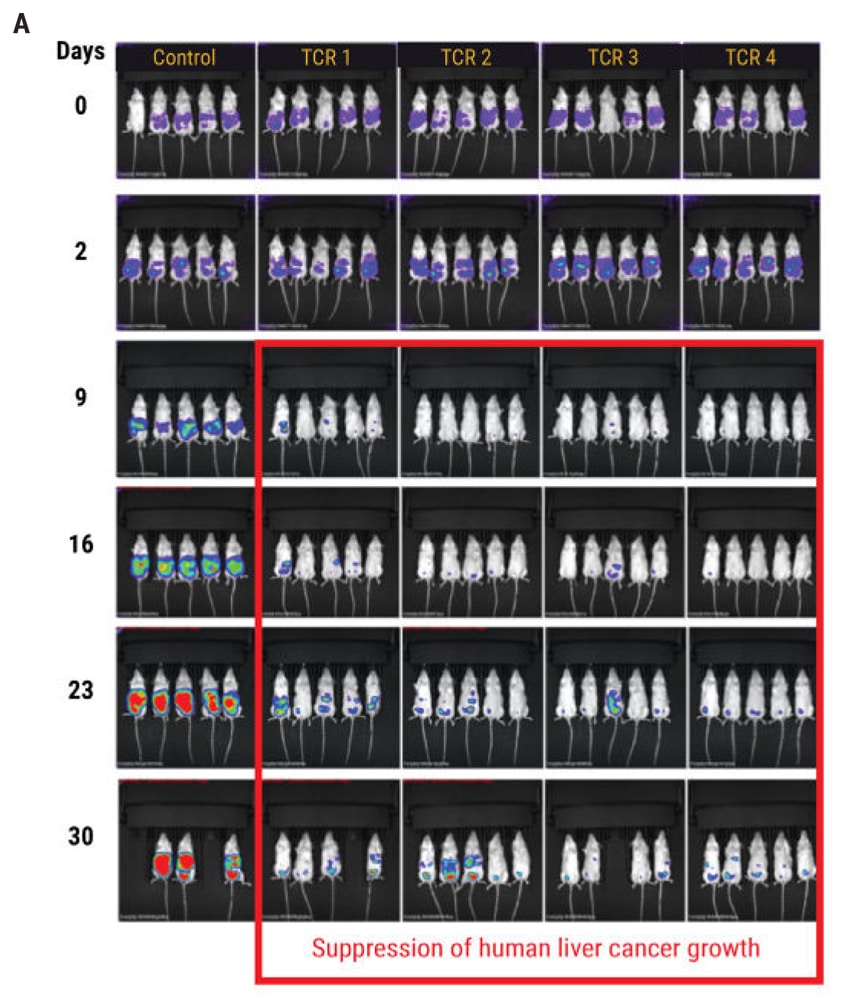
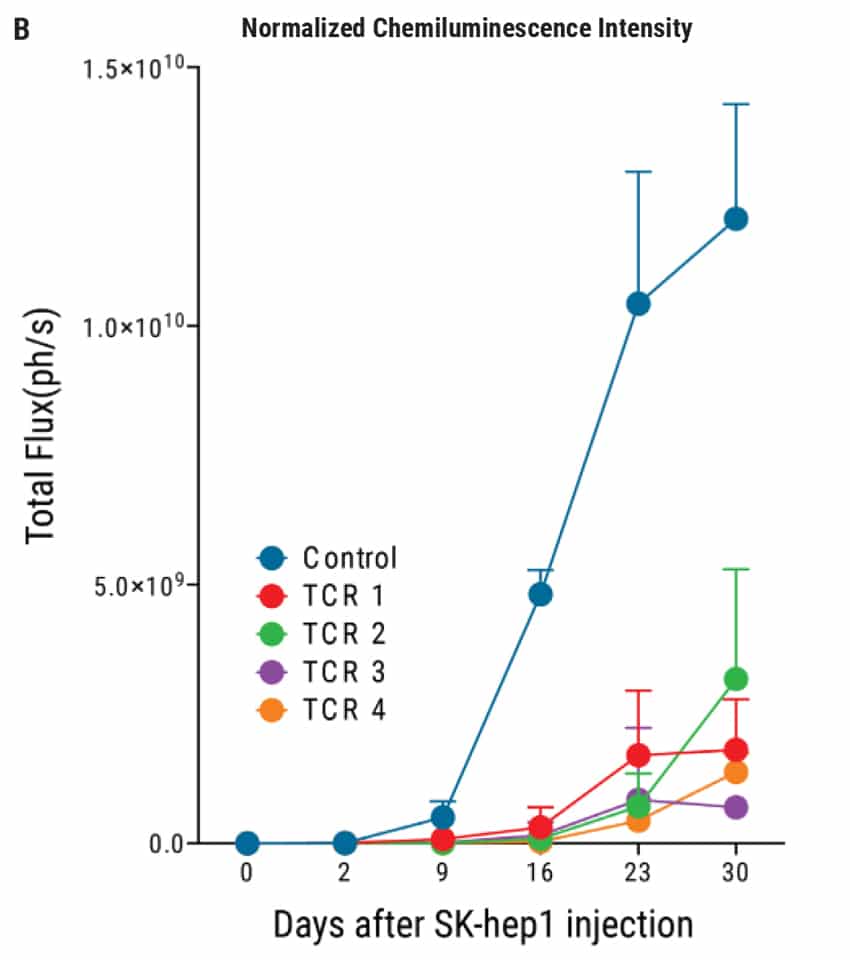
Figure 4: In vivo efficacy of TCR-transduced iPSC-derived T cells. A) Tumor formation was induced through the intraperitoneal injection of 1x104 luciferase-transduced SK- Hep1 liver cancer cells into NSG mice followed by injections of T cells on day 2, 4, 7, 9, 11, and 14. The tumor size was monitored by in vivo bioluminescence imaging. B) Results from Figure 4A are displayed as the total average flux of each TCR-tranduced T cell-treated mouse group. In all four TCR treated groups, the tumor development was inhibited by day 9, compared with control mice. Subsequent injections of TCR T cells resulted in continued suppression of tumor development for up to 30 days. Chemiluminescent intensity of the treated mice was normalized to control mice at each time point.
Summary
- MaxCyte® Electroporation® Technology enables delivery of diverse payloads including DNA piggyBac transposons for stable cell lines without viral vectors.
- The high efficiency of MaxCyte electroporation allows for rapid optimization of plasmid promoter selection, DNA concentration, and DNA ratio.
- Non-viral engineering enables creation of stable TCR-expressing iPSCs with high efficiency and shortened timelines.
- MaxCyte engineered iPSCs differentiate into functional T cells with antigen-specific activity in vitro and in vivo.
Corresponding Author: James Brady; [email protected] MaxCyte, Inc., Tel: (301) 944-1700





