Poster
Efficient, Scalable Manufacturing of Virus-Like Particles for the Delivery of Gene Editor Ribonucleoproteins Using a GMP-Compatible Electroporation Platform
Abstract
Genome editing tools such as CRISPR-Cas9 nucleases, base editors, and prime editors hold tremendous promise for treating human diseases by being able to specifically modify a targeted region of the DNA genome. However, there are limited in vivo delivery options that are safe, efficient, and transient. Moreover, viral vectors such as AAV are hampered by limited cargo size capacity. Virus-like particles (VLPs) offer a solution to these problems. VLPs are derived from retroviral structural proteins, which can be engineered to specifically package a cargo of interest. They are safer than traditional viral vectors because they lack a viral genome but can utilize the traditional virus delivery machinery to target and enter cells. Recently, VLPs have been reported to efficiently package base editors and prime editors for delivery into mice at therapeutically relevant levels. To realize the potential of VLPs for in vivo delivery, alternative methods to scale up their manufacturing will be critical for future clinical applications. Here, we utilized the MaxCyte® ExPERT GTx™, a GMP-compatible electroporation instrument, to manufacture VLPs— packaged with CRISPR-Cas9 or base editor (BE) ribonucleoproteins (RNPs) for genome editing in target cells — human immortalized HEK293T cells and primary immune cells. We found that electroporation consistently produced significantly higher yields of functional VLPs compared to a commercially available transfection reagent, with greater than 10-fold improvement in p30 titer when using an optimized electroporation protocol. We also demonstrate effective genome editing at the B2M locus, with both CRISPR-Cas9 and BE RNP-packaged VLPs. Furthermore, the production of VLPs using electroporation exhibited favorable production kinetics compared to other transfection methods, enabling a shorter VLP manufacturing process. Finally, we demonstrated the scalability of VLP production across a 15-fold volume range with minimal reoptimization. In summary, our results show that electroporation is a viable means for consistent, efficient, and scalable manufacturing of VLPs for gene-editing applications and has high promise to address needs for future clinical and commercial VLP manufacturing.
Figure 1: Experimental workflow for VLP preparation and assessment of VLP yields
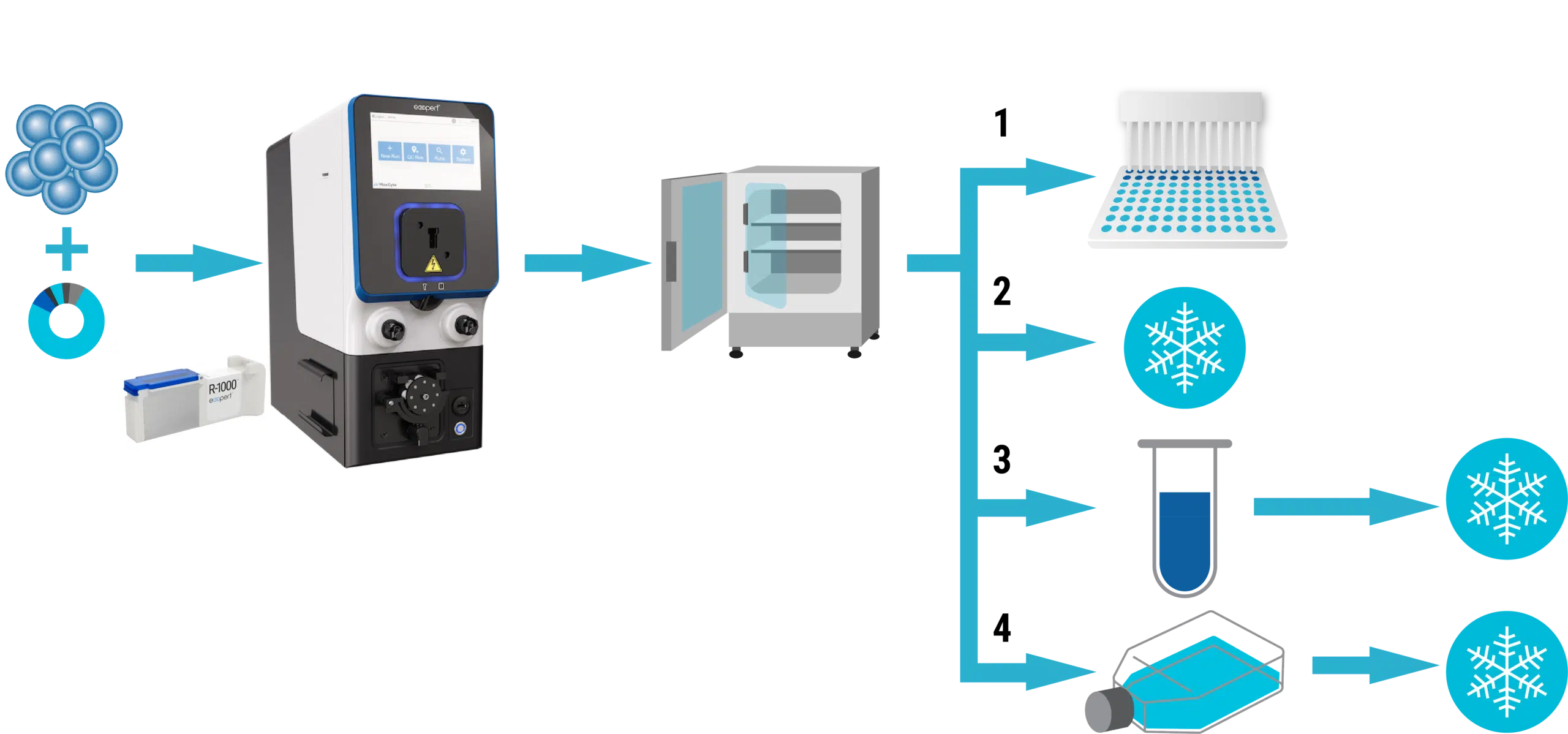
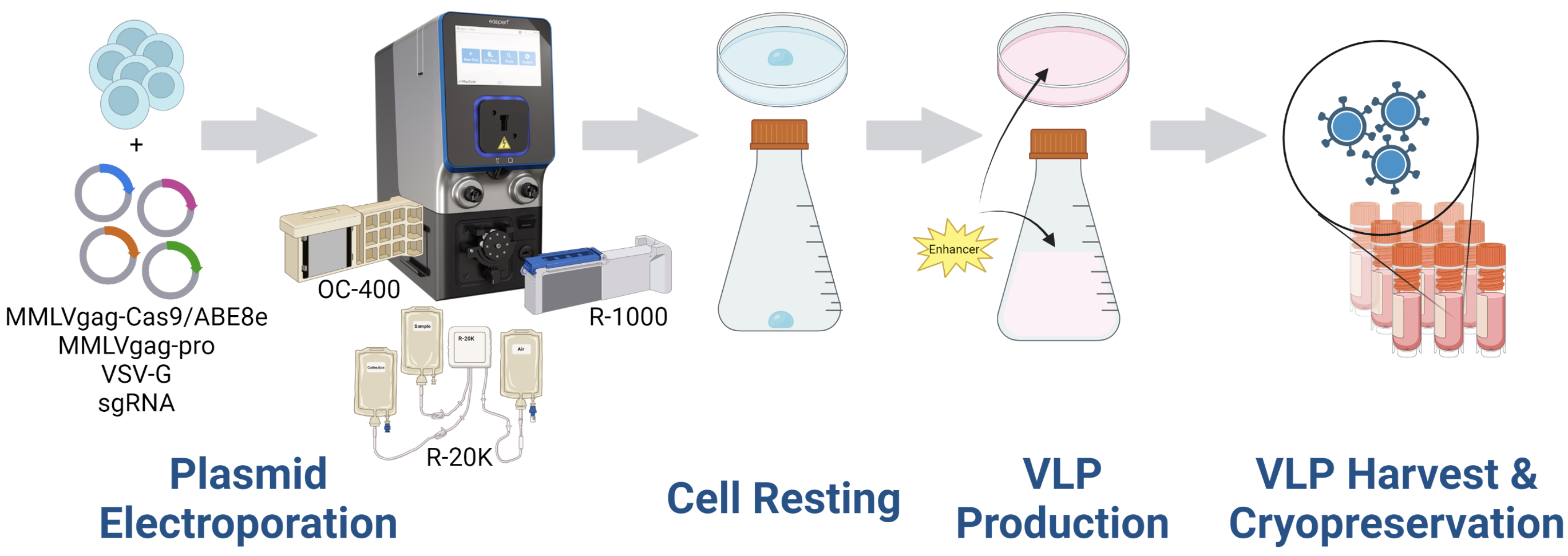
A – Experimental workflow for VLP preparation
B – Assessment of VLP yields
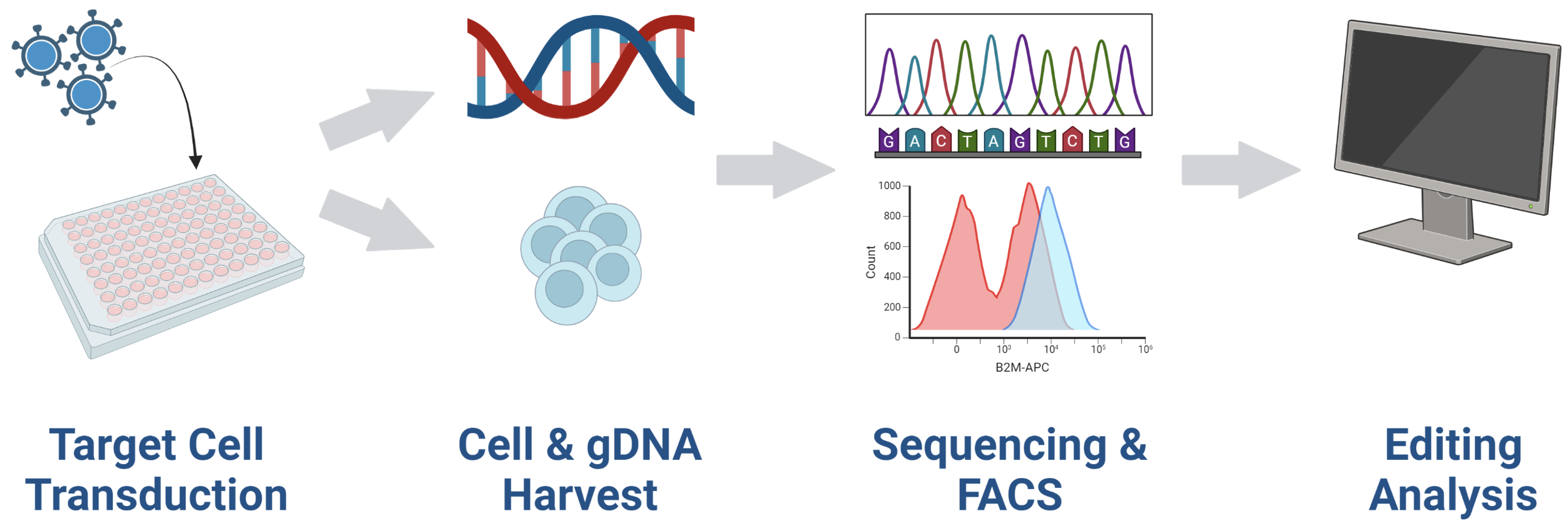
Figure 1 A) Adherent or suspension HEK293 cells were resuspended in MaxCyte electroporation buffer with a DNA plasmid mix (containing MMLVgag-Cas9/ABE8e, MMLVgag-pro, VSV-G, and sgRNA). Cells were electroporated in OC-400, R-1000, or R-20K processing assemblies with the appropriate pre-loaded program. Electroporated cells were rested in empty culture vessels for 30 to 40 minutes at 37º C (5% CO2, 80% humidity) and then 10-20 mL of complete media was added. Culture media was collected 1- or 2- days post electroporation. For direct comparison of production methods in adherent HEK293, electroporated (1.5x107 cells) or chemically transfected cells (~7.5x106 cells) were cultured in 10 cm dishes according to the recommended protocol. VLP harvest volume was 10 mL from each method. For direct comparison in suspension HEK293, starting cell numbers and culture vessels are identical. VLPs were precipitated from the clarified supernatant using Lenti-XTM concentrator, resuspended in 100 μL of PBS, and stored in aliquots at -80ºC. B) VLPs were used to transduce HEK293 or primary immune cells and genomic DNA from VLP-transduced cells was harvested for Sanger sequencing after 3-5 days. DNA sequences were analyzed by the Synthego ICE Analysis tool to determine the rate of insertions and deletions (% indels) or EditR to determine the rate of base substitutions. Cells were harvested 3-5 days post-transduction for flow cytometric analysis.
Figure 2: Optimization of VLP production in electroporated adherent HEK293 cells
A
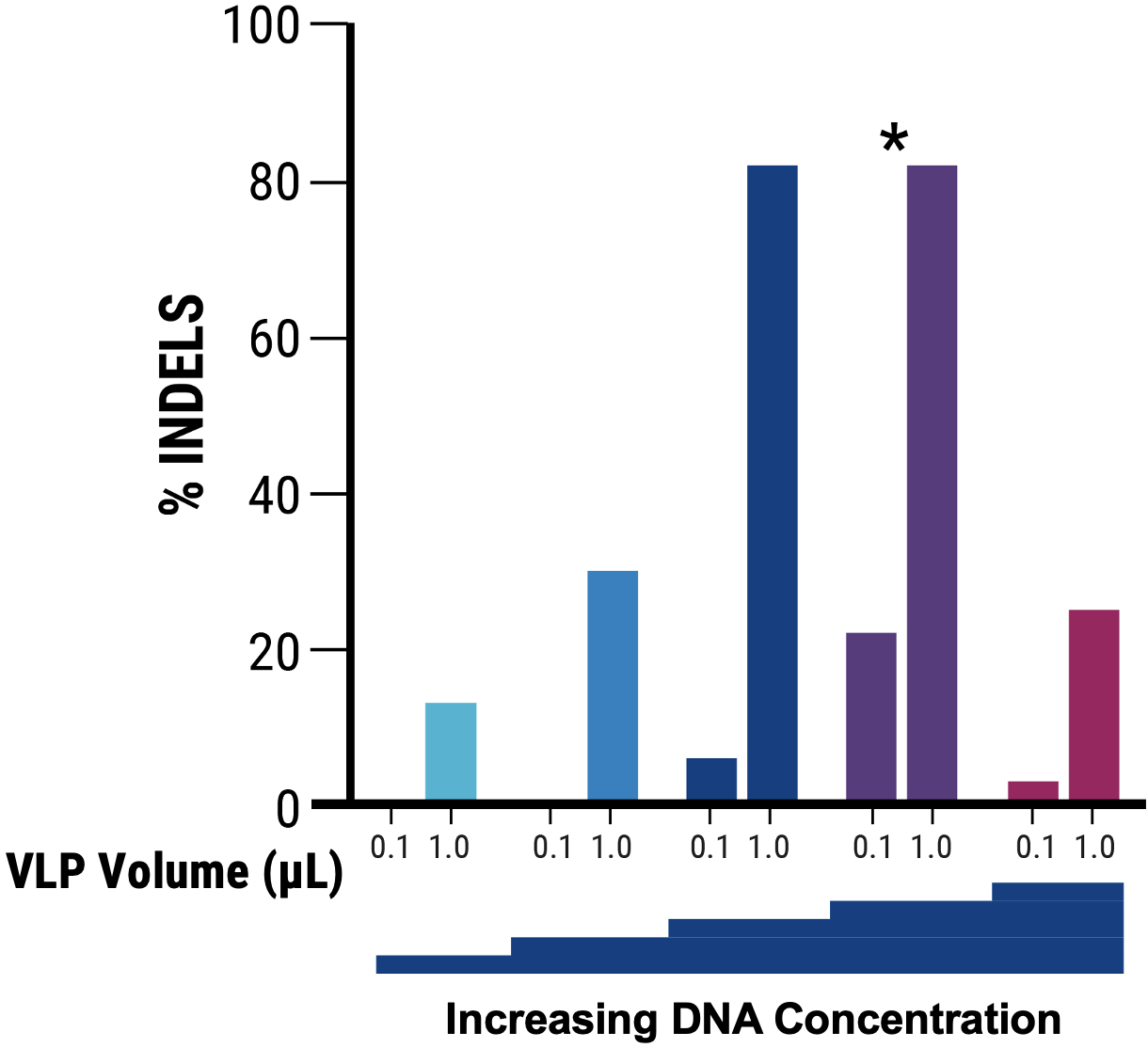
B
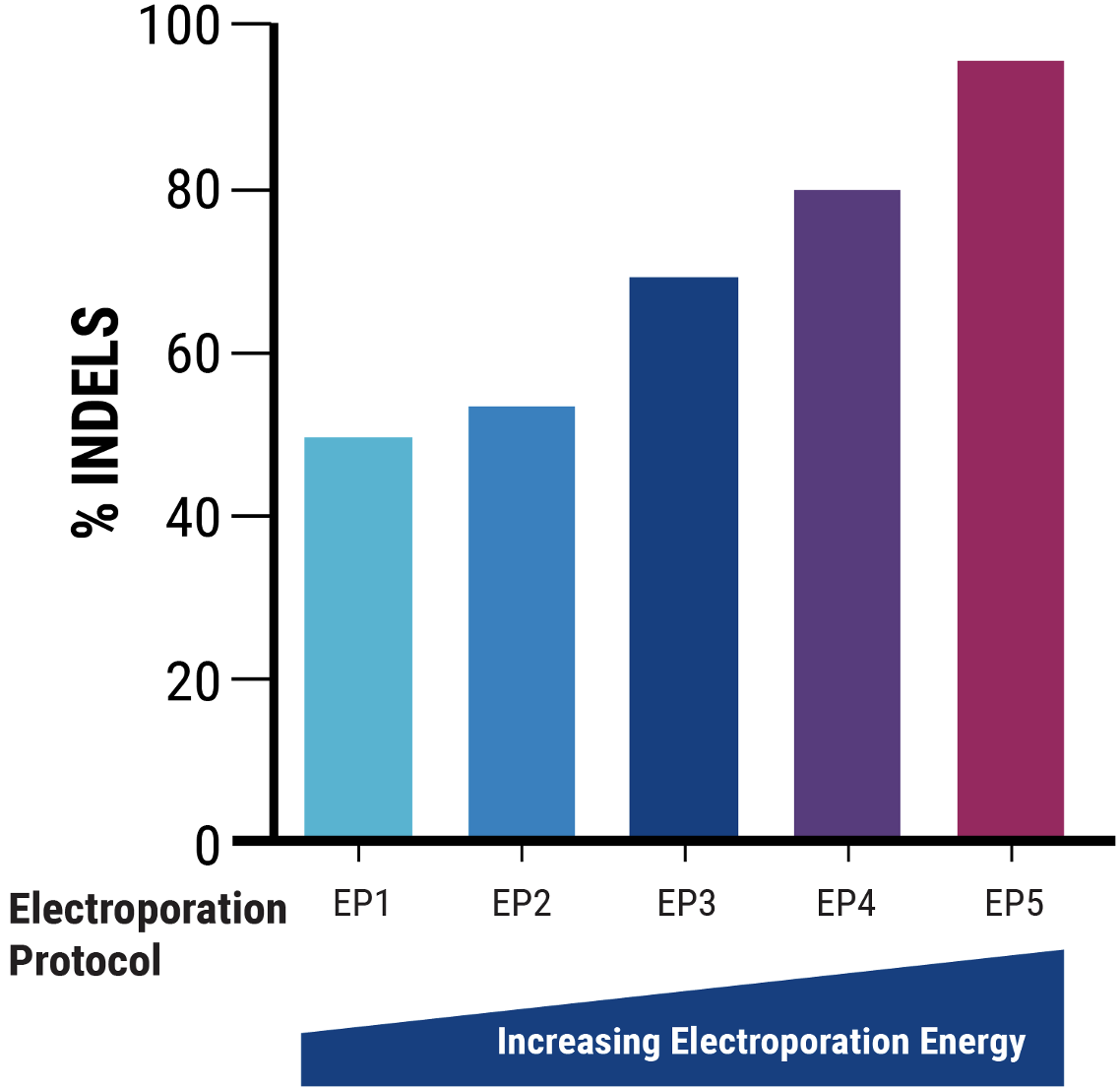
C
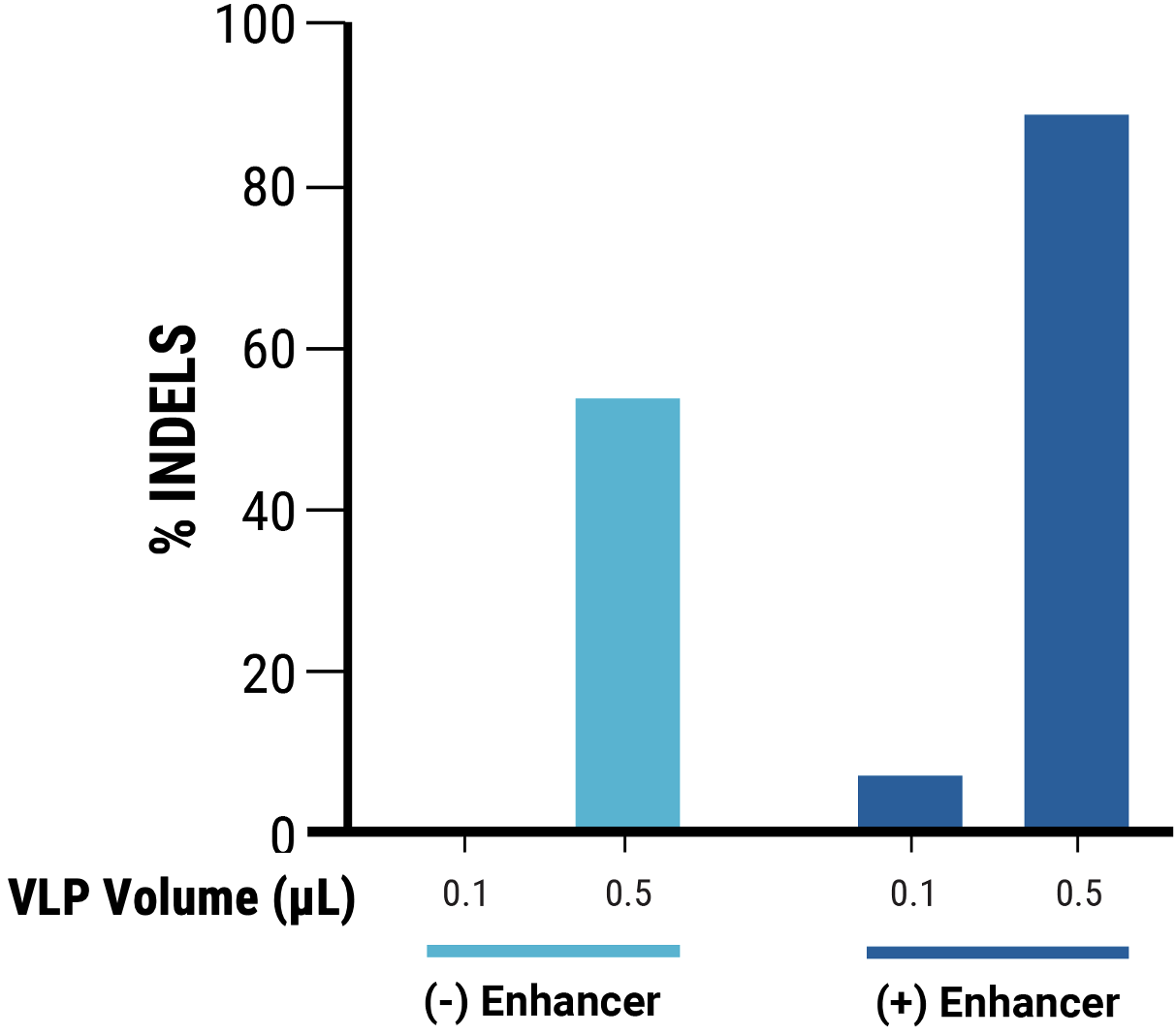
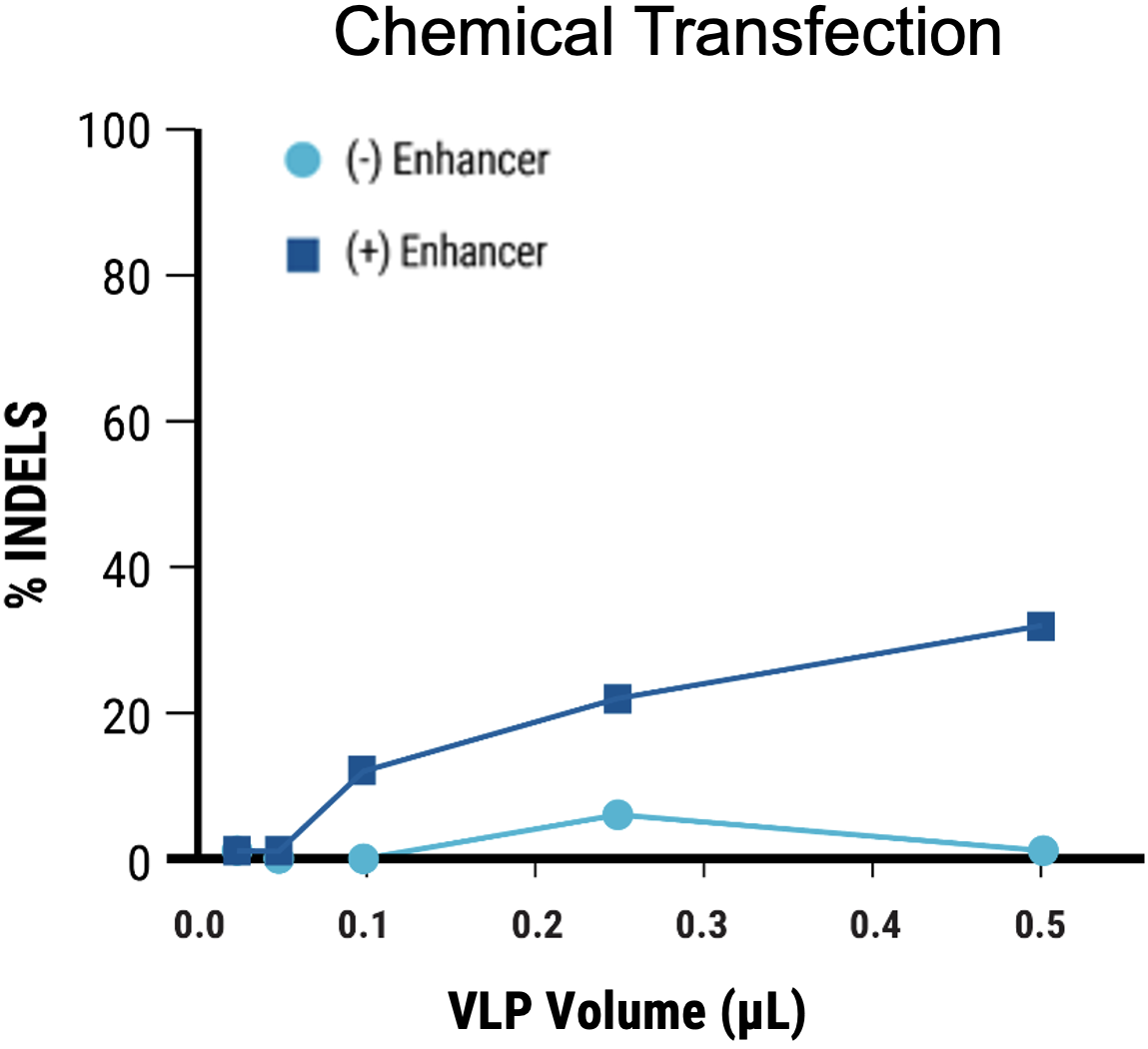
Figure 2 A) Identification of optimal pDNA concentration (*) for VLP production to obtain the highest nuclease-induced genome editing at the HEK3 locus of VLP-targeted cells. B) Increased electroporation energy used for delivering VLP-producing plasmids resulted in higher indels by VLP-delivered nucleases. C) The addition of a chemical enhancer during the VLP production process (EP4) increased the genome editing activity of VLP-delivered nucleases at comparable volume.
Figure 3: Electroporation produces more VLP-mediated editing activity than chemical transfection in adherent HEK293 cells
A

B
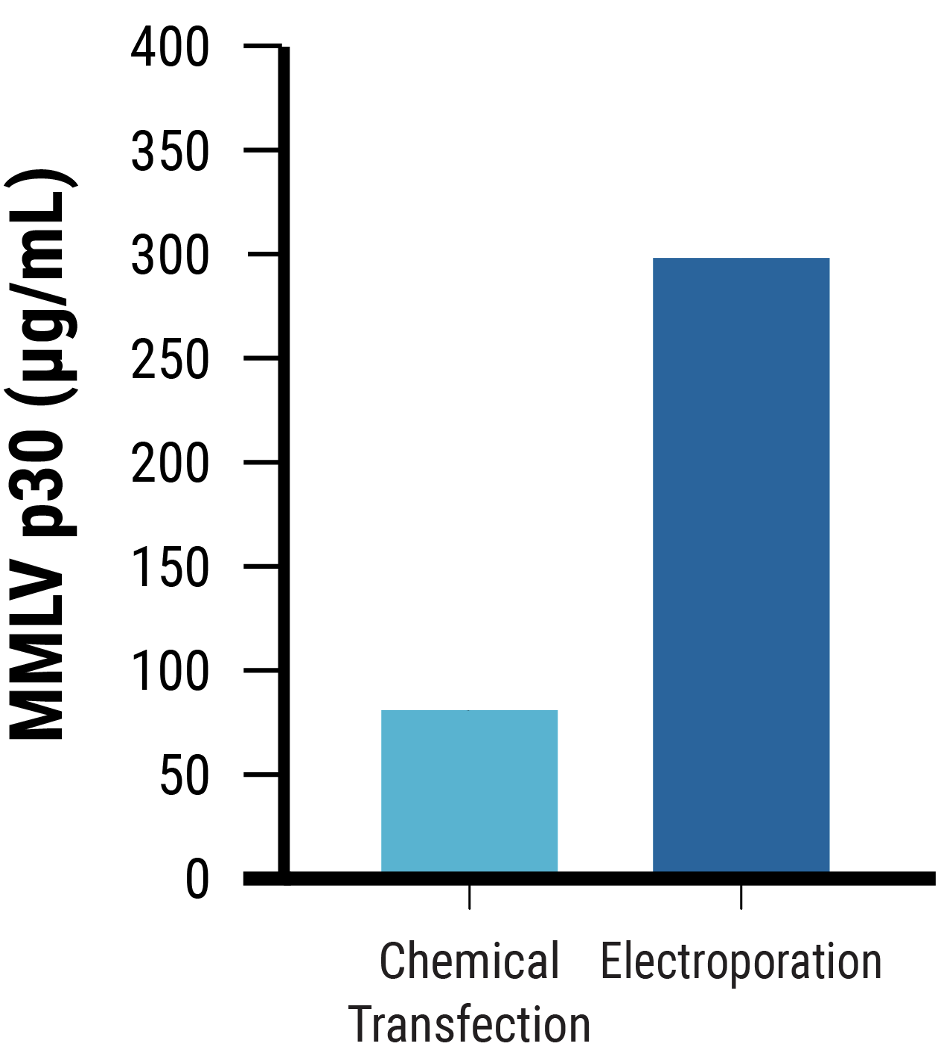
Figure 3 Adherent HEK293 cells were transfected with the optimized electroporation procedure (EP5) or chemical transfection; no enhancer was added. VLPs were harvested on day 2 post-transfection, and editing activity was determined. A) VLP editing activity (HEK3) from electroporated cells was higher than from chemically transfected cells. B) MMLV p30 ELISA demonstrated that electroporated cells produced ~4x higher MMLV p30 levels than chemically transfected cells.
Figure 4: Electroporation enables faster production of VLP activity than chemical transfection in adherent HEK293 cells
A
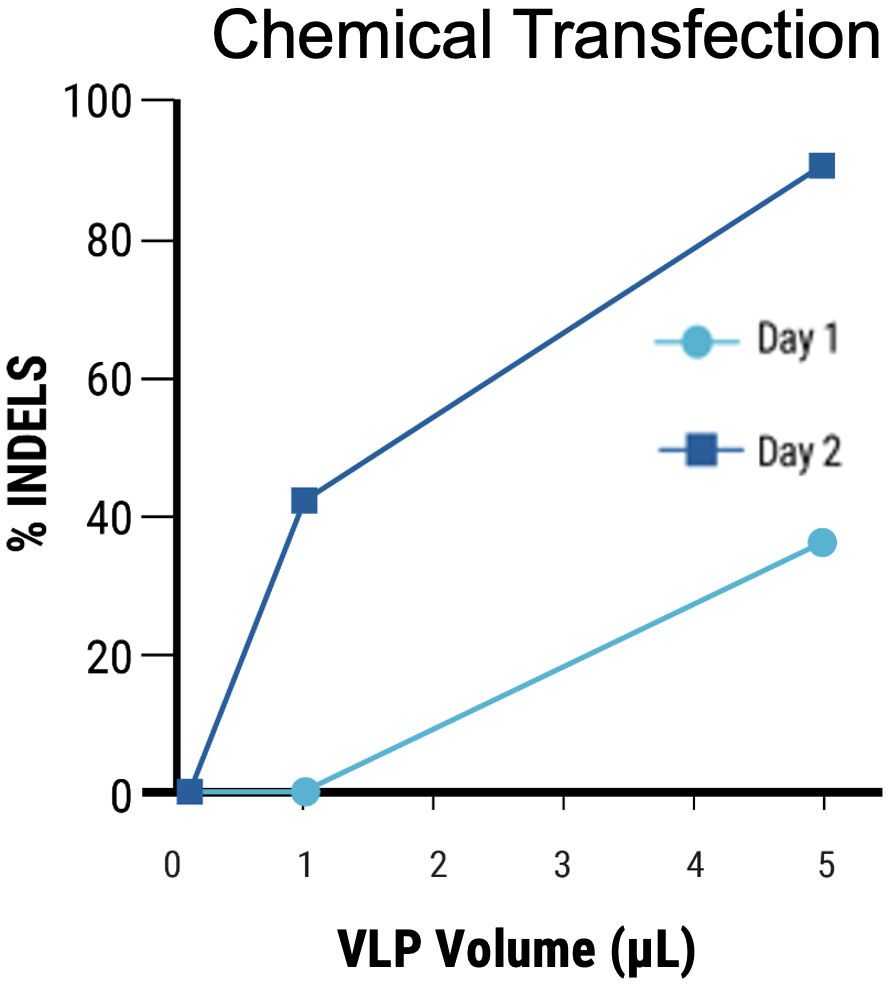
B
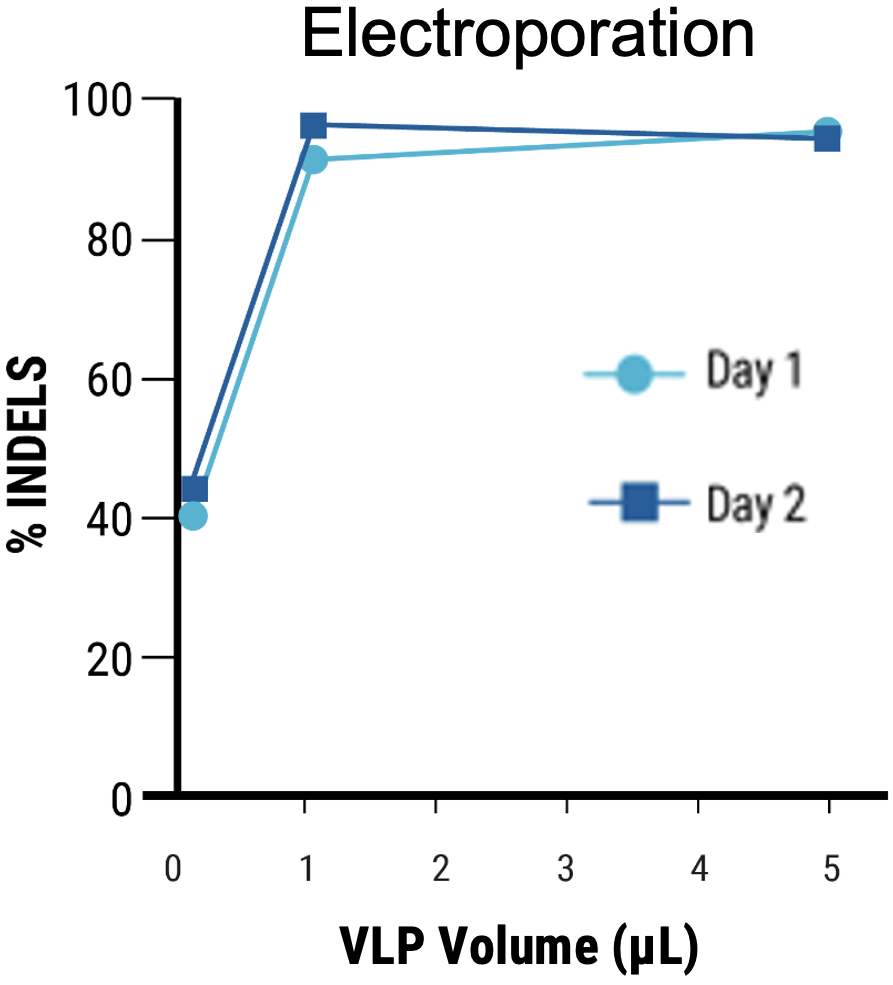
C
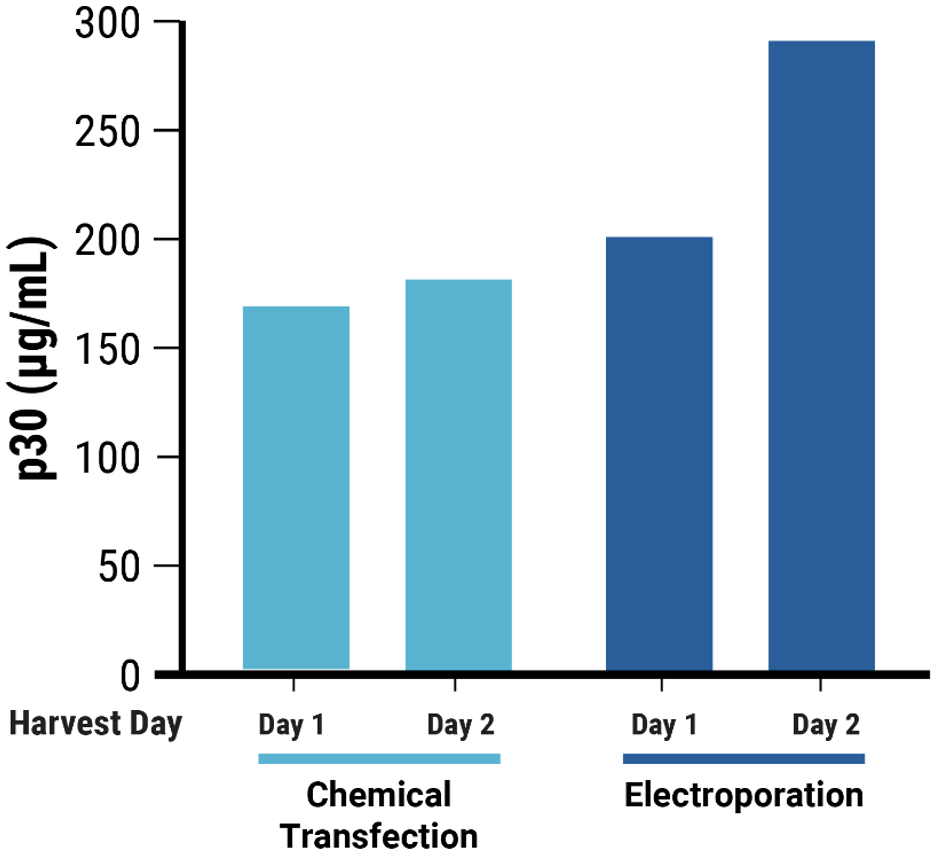
D
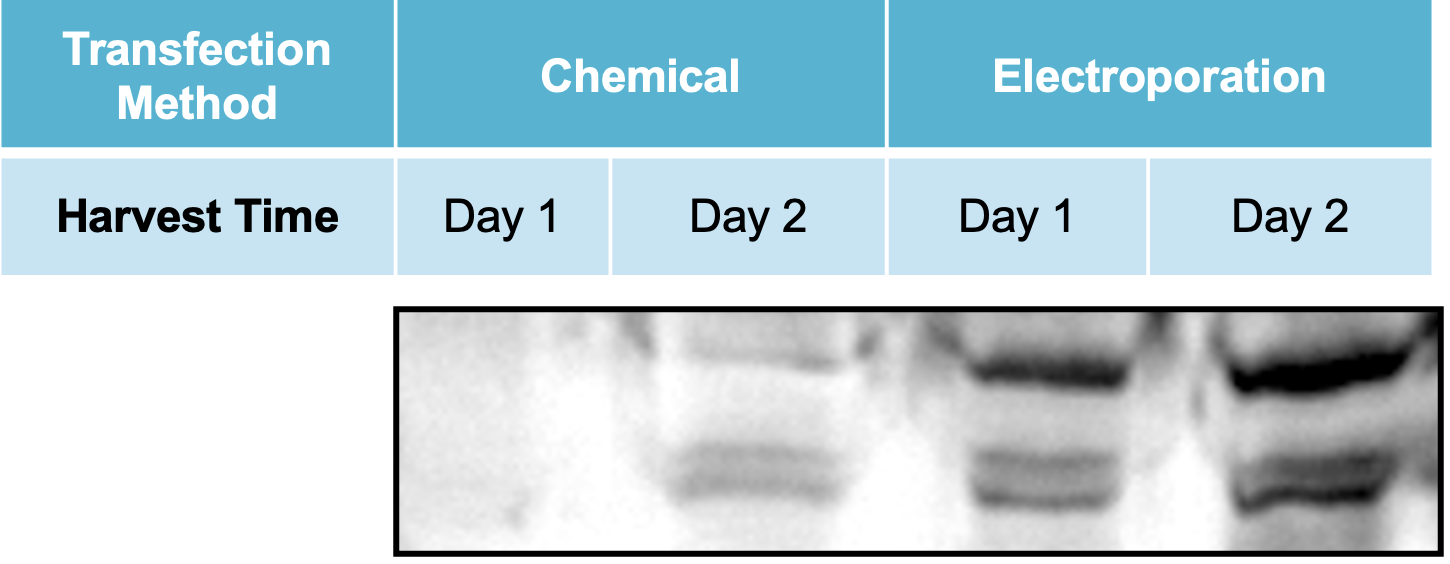
Figure 4 Adherent HEK293 cells were transfected following the optimized electroporation procedure or with chemical transfection. VLPs were harvested on day 1 and day 2 posttransfection. A) Chemically transfected cells produced consistently lower VLP editing activity (HEK3) than B) electroporated cells. C) MMLV p30 ELISA showed higher p30 in the VLP harvest from electroporated cells than chemically transfected cells. D) Anti-Cas9 western blot demonstrated higher, faster Cas9 incorporation into the VLP harvest from electroporated cells compared with chemical transfection. VLPs were loaded at the same concentration based on p30 ELISA analysis.
Figure 5: Comparison of VLP editing activity following electroporation or chemical transfection in suspension HEK293 cells
A
B
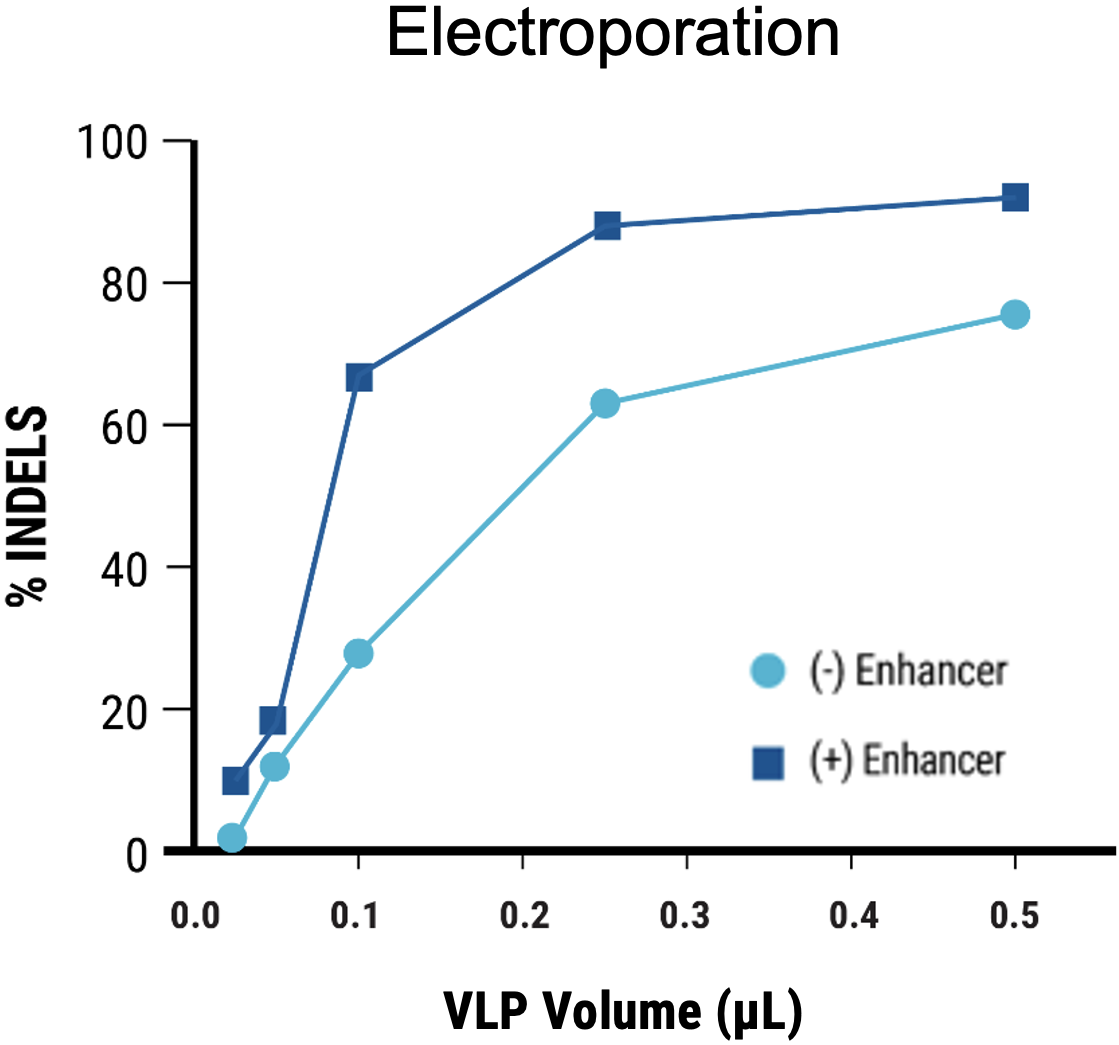
C
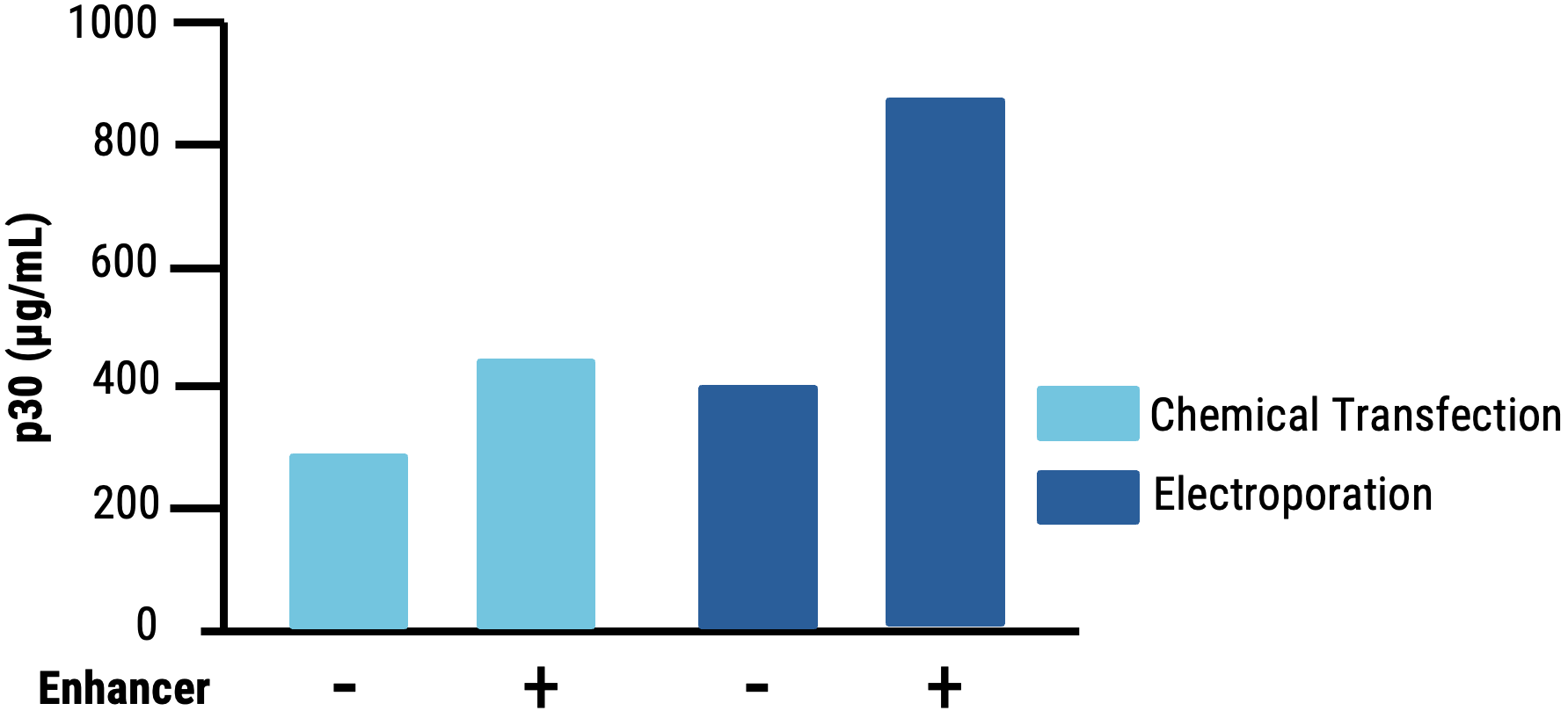
Figure 5 Suspension HEK293 cells were transfected by electroporation (EP4) or with chemical transfection. VLPs were harvested on day 1 post-transfection. VLPs produced in suspension HEK293 cells following chemical transfection A) resulted in lower editing efficiency (HEK3) compared to electroporation. B) The addition of an enhancer during VLP manufacturing produced VLPs with improved editing activity. C) MMLV p30 ELISA showed higher levels of p30 in the VLP harvest from electroporated suspension HEK293 cells than chemically transfected cells.
Figure 6: Electroporation enables efficient packaging of base editor RNPs into VLPs
A
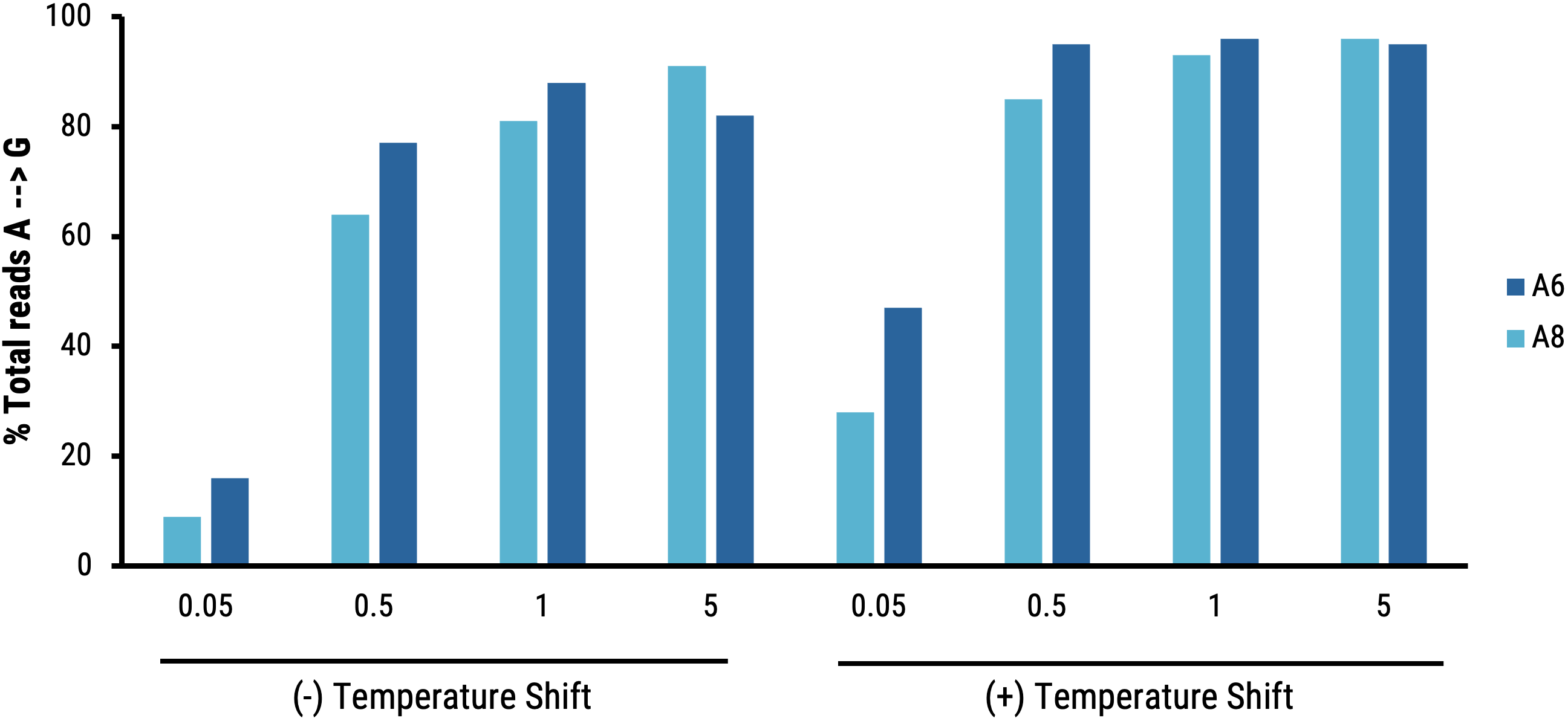
B
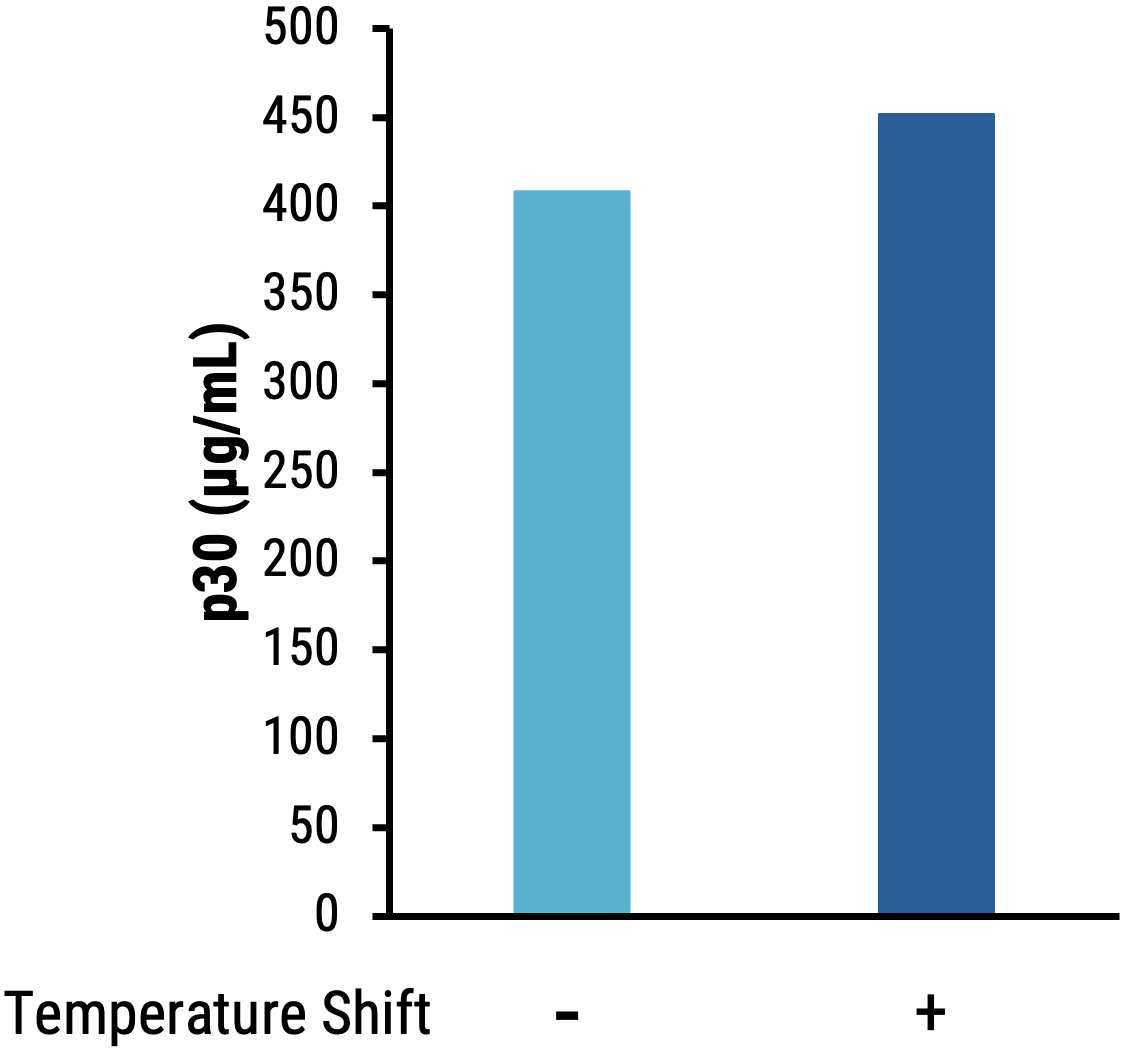
Figure 6 Suspension HEK293 cells were transfected with electroporation (EP5) and enhancer was added to production media. Culture temperature was shifted and VLPs were harvested on day 1 post-transfection, and editing activity (base substitutions) was determined in HEK293T. A) Shifting producer cell culture improves ABE8e-VLP editing activity (HEK3). B) MMLV p30 ELISA demonstrates that the temperature shift also improves p30 titer.
Figure 7: Primary immune cells are efficiently edited with VLPs produced via electroporation
A
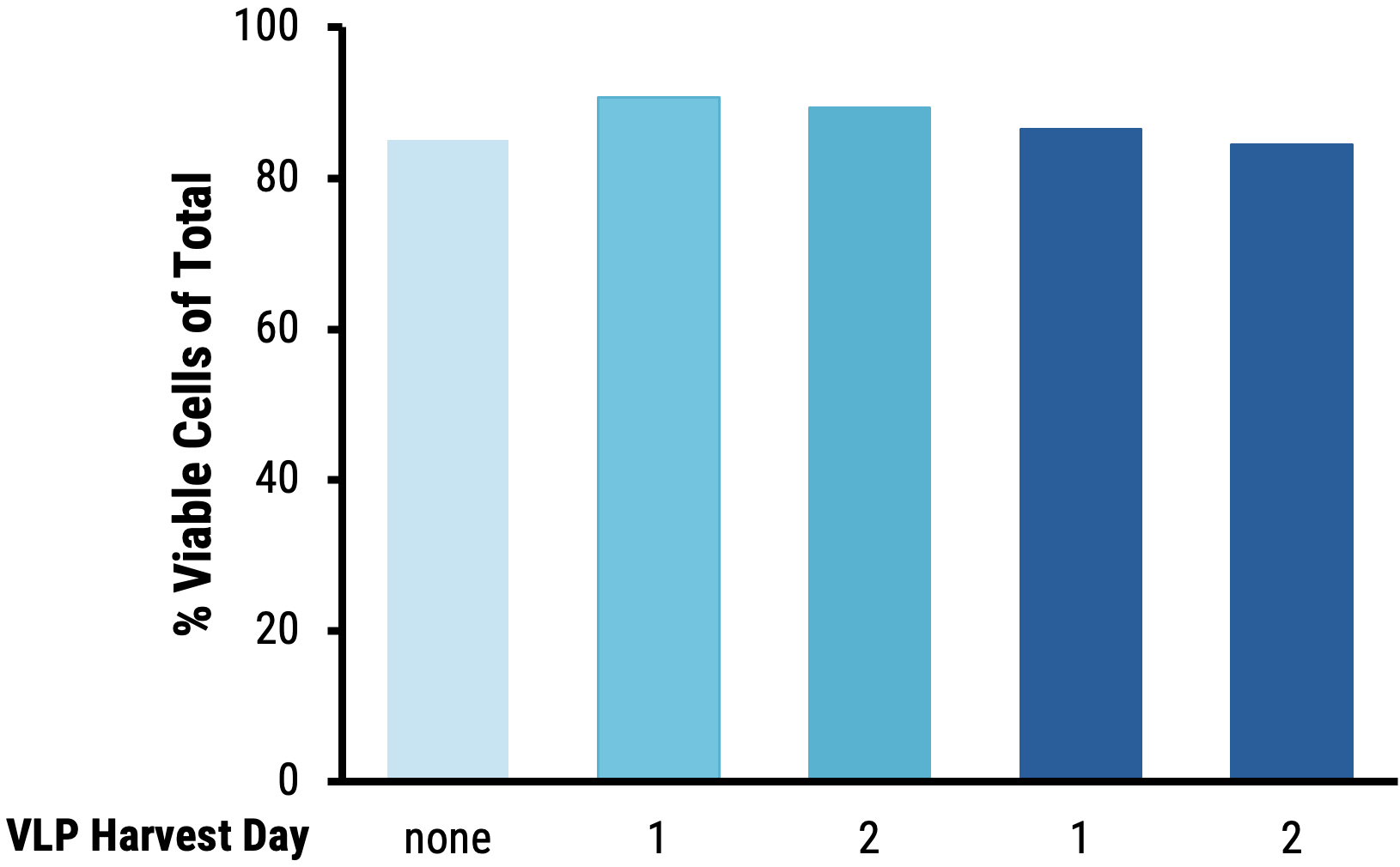
B
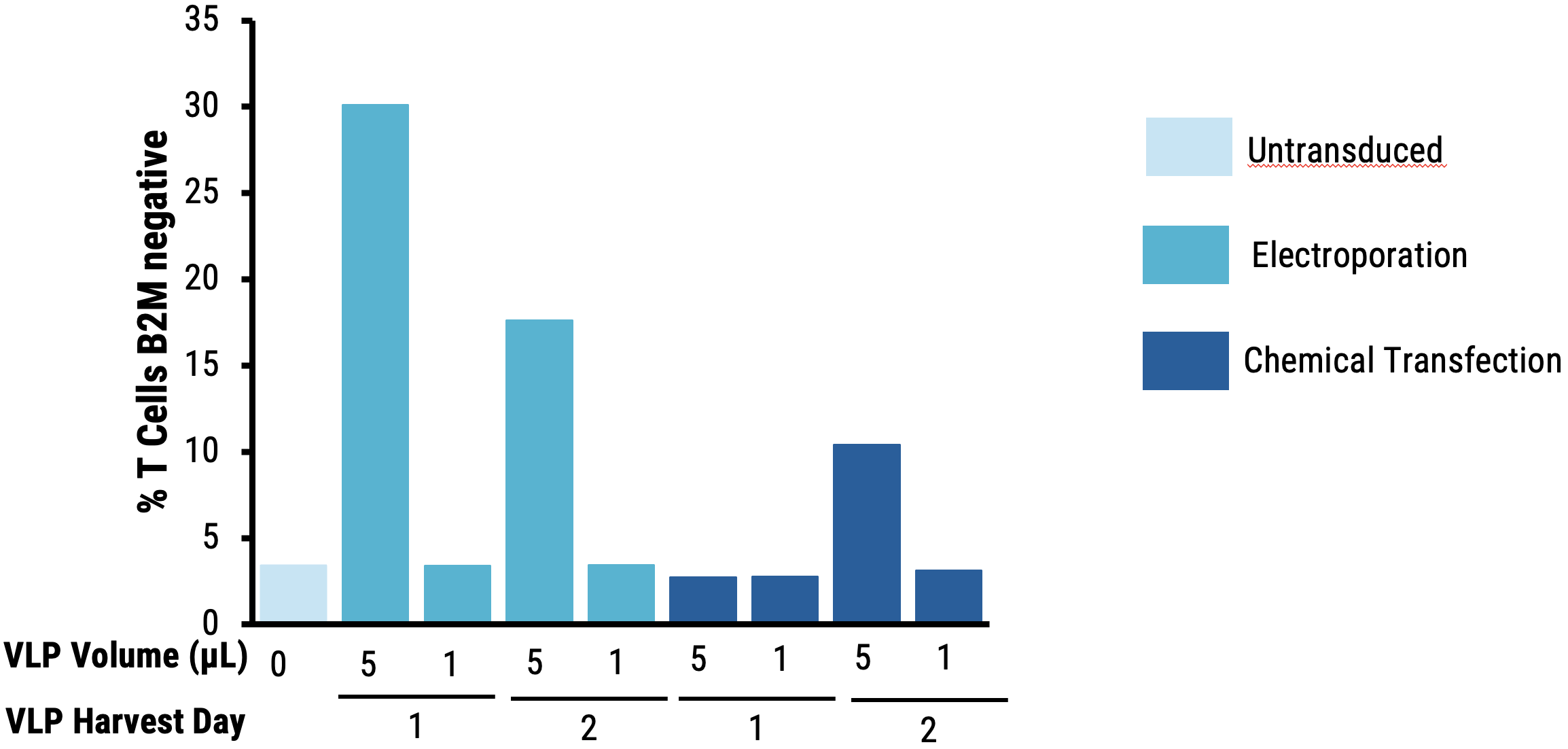
Figure 7 Suspension HEK293 cells were transfected by electroporation (EP5) or with chemical transfection and enhancer was added to production media and culture temperature was shifted. VLPs were harvested on day 1 or 2 post-transfection. Resting T cells were isolated from PBMCs and activated for 48 hours. Cells were transduced on a RetroNectin®-coated plate for 2 days, then expanded and re-transduced. On day 5, cells were harvested and analyzed for cell viability (AO/PI) and B2M surface expression (FACS). VLPs produced in suspension HEK293 cells following electroporation A) do not negatively affect T cell viability 5 days post-transduction at the highest dose (5 μL) and B) disrupt B2M expression in T cells more efficiently compared to VLPs produced following chemical transfection.
Figure 8: Scalable VLP production with a GMP-compatible electroporator in suspension HEK293 cells
A
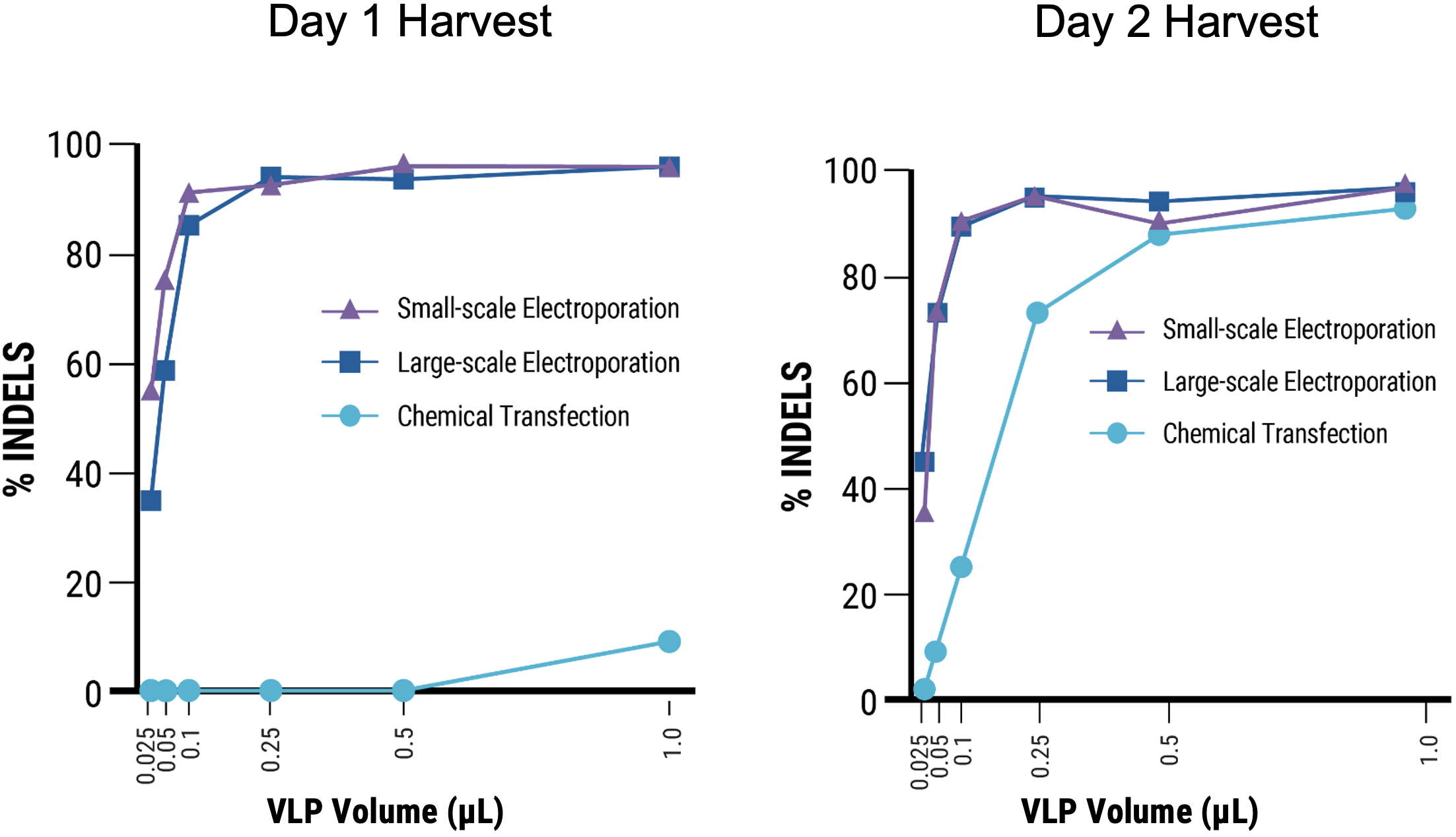
B
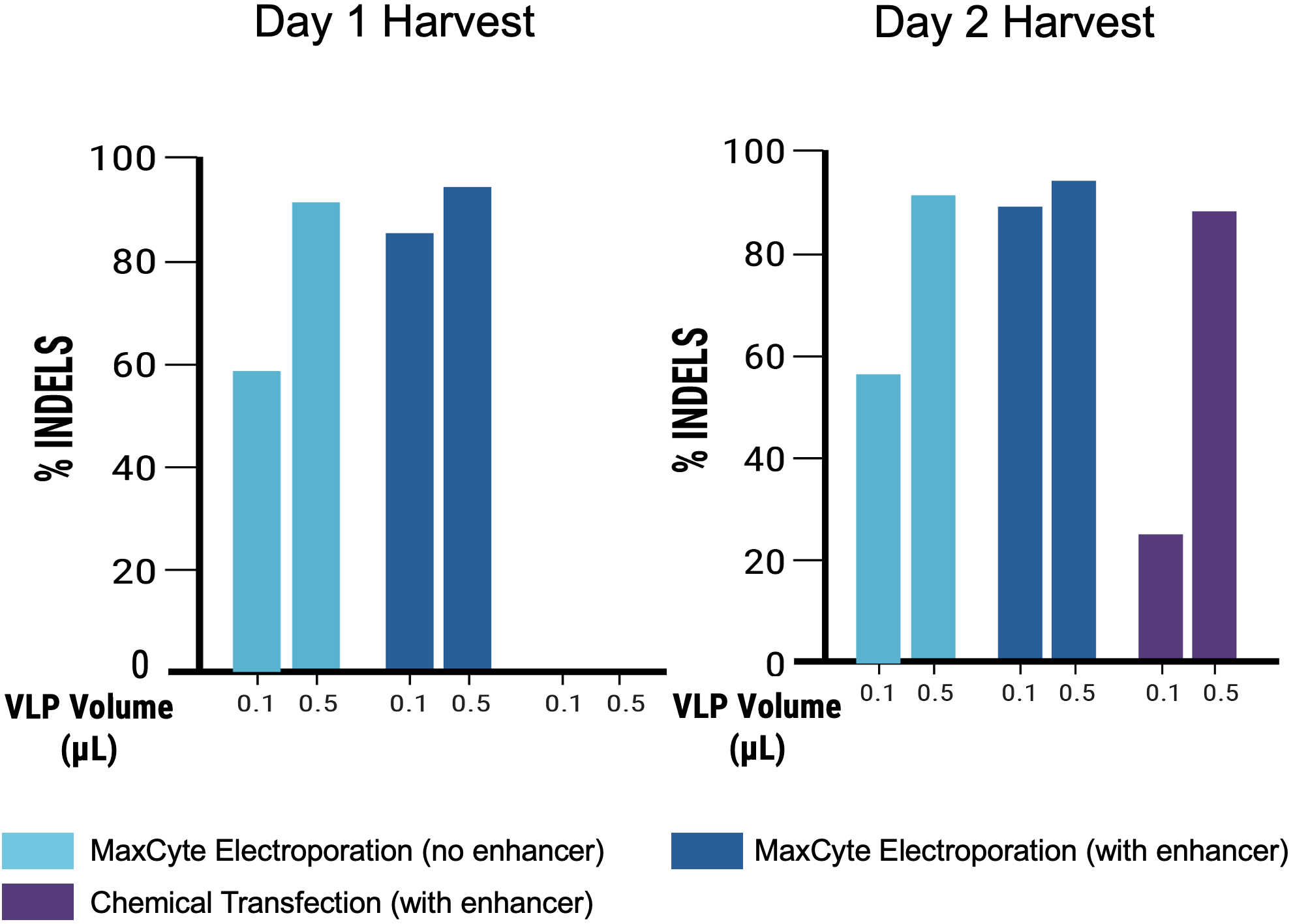
C
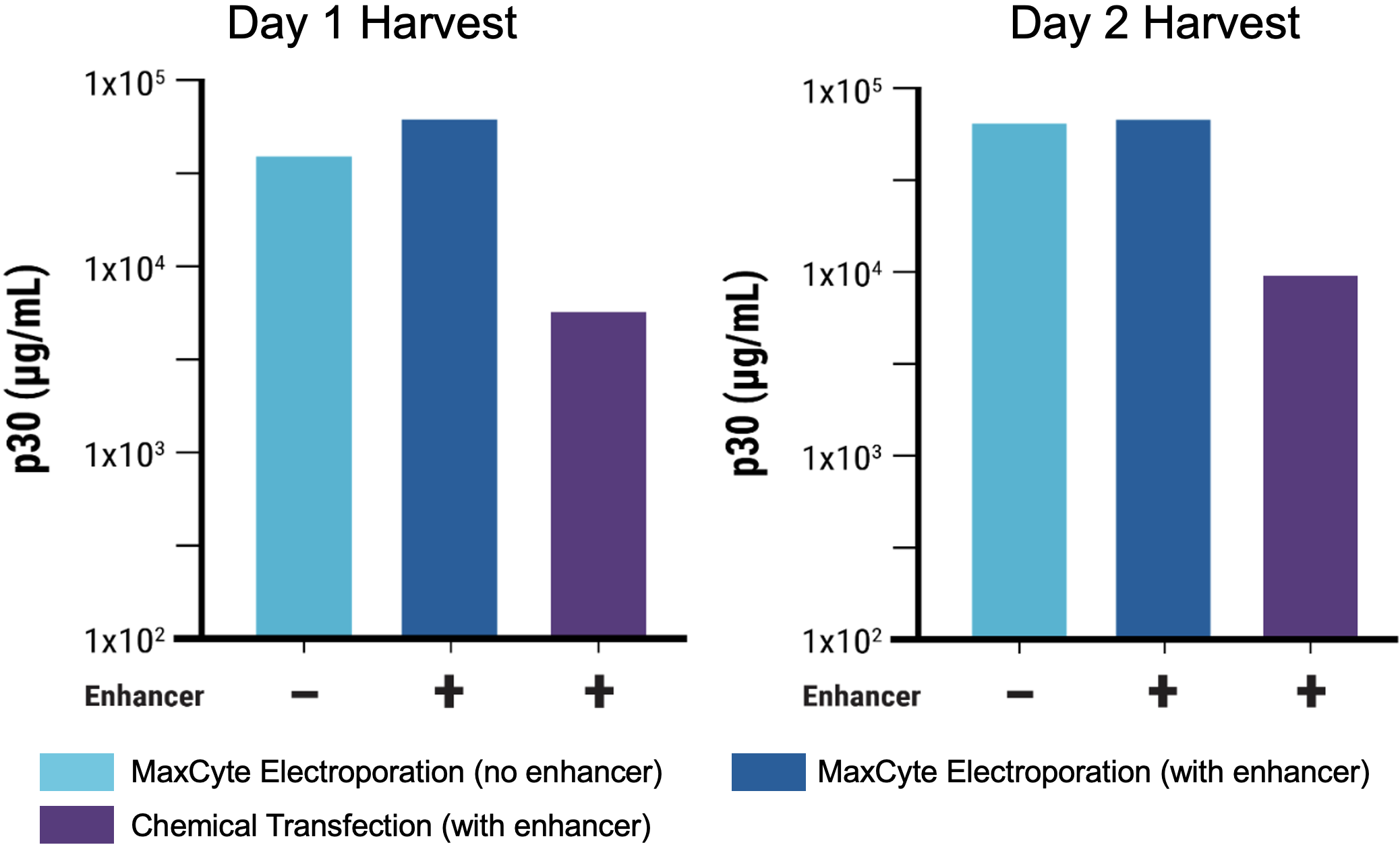
D
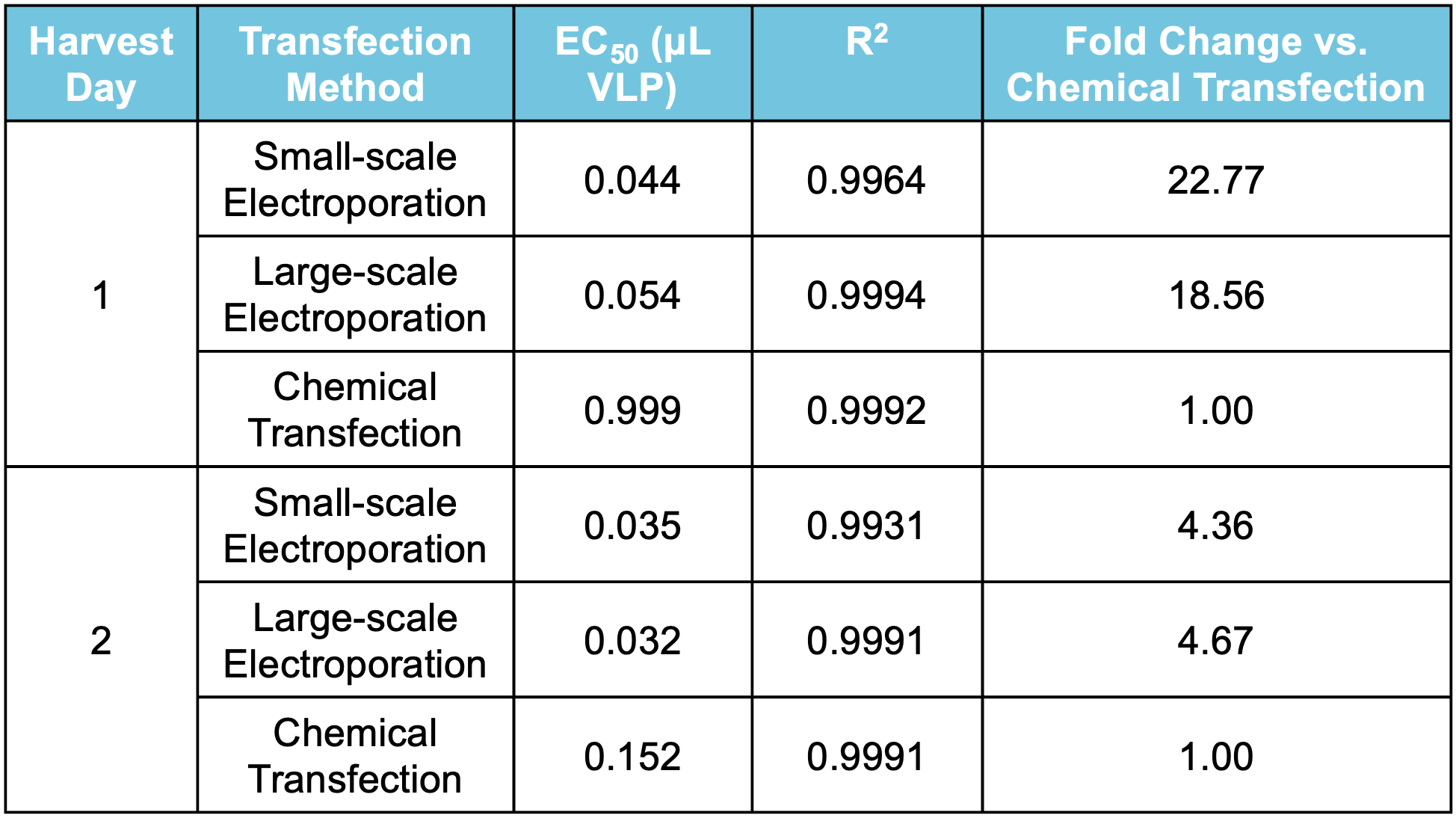
Figure 8 Suspension HEK293 cells were transfected via electroporation (EP5) or chemical transfection, chemical enhancer was added where indicated. VLPs were harvested on day 1 or 2 post-transfection. A) Transfection with MaxCyte’s flow electroporation® technology (1x109 cells) produced similarly high VLP editing efficiency as small-scale static electroporation (1.2x108 cells), using the same optimized conditions. Electroporation produced higher VLP editing efficiency than chemical transfection (1.2x108 cells). Following electroporation, VLPs can be harvested on day 1 post-transfection. B) Electroporation, with and without enhancer, produced higher VLP editing activity than chemical transfection. C) MMLV p30 ELISA demonstrated that electroporated cells yielded >10x higher p30 expression on day 1 post-transfection, and ~7x higher expression on day 2 compared with chemical transfection. D) EC50 measurements demonstrate approximately 18- to 22-fold increase in potency of VLPs produced with electroporation harvested on day 1, and 4-fold increase on day 2 versus chemical transfection.
Summary
- Demonstrated scalable GMP-compatible transfection of 1 billion cells with MaxCyte’s Flow Electroporation® technology
- MaxCyte electroporation is an efficient, scalable method for producing functional VLPs in both adherent and suspension HEK293 cells
- Electroporation produces higher VLP-mediated editing activity, on a shortened timeline, than chemical transfection
- Higher p30 and Cas9 yields were obtained in the VLP harvest from cells transfected by MaxCyte electroporation than chemical transfection
- MaxCyte’s electroporation yielded rapid VLP production, enabling a 1-day manufacturing process
- ABE8e-VLPs produced via electroporation efficiently edit at the B2M locus in activated T cells, resulting in viable B2M-negative T cells
- MaxCyte electroporation is a simple, time-saving alternative to chemical transfection for VLP production that can be used in clinical applications