Application Note
Keys to Successful Cell-Based Assay Development with Scalable Electroporation
Introduction
Drug discovery and development is a costly, time-consuming process with a high risk of failure. One approach to save time and mitigate risk is to increase the use of cell-based assays as an alternative to biochemical assays. Cell based assays (2D and 3D) enable interrogation of a target in a physiological context and have the potential to be used in all phases from target discovery up to preclinical development.
Cell-based assays have historically relied on stable cell lines; however, their development is expensive and labor-intensive and imposes constraints on the cell types that can be used. The process becomes more challenging when working with toxic proteins.
With the right technology, transiently transfected Assay Ready Cells can be a time-saving, cost-effective alternative to stable cell lines.
MaxCyte® is a pioneer in electroporation technology for mammalian cell engineering, combining optimized protocols, reagents and consumables to deliver reproducible performance with minimal cell disturbance. MaxCyte’s static and Flow Electroporation® enable high transfection efficiency in virtually any cell type, with seamless scalability from 75,000 to 200 billion cells.
MaxCyte’s electroporation technology can be used to produce Assay Ready Cells, addressing the time and cost of assay development with stable cells. The use of Assay Ready Cells facilitates the expression of toxic proteins and enables assay development in physiologically relevant cell types, including primary cells, stem cells and cells of hematopoietic origin.
MaxCyte’s electroporation platform was developed to have minimal impact on cells. Here we present some factors impacting cell viability and discuss how to optimize conditions for Assay Ready Cell production and successful cell-based assay development.
DNA Preparation
DNA quality affects both transfection efficiency and cell viability. Plasmid DNA can be prepared using commercially available endotoxin-free kits; however, DNA should be resuspended in water since EDTA can interfere with electroporation buffer conductivity. The OD260/280 ratio should be greater than 1.8, and agarose gel electrophoresis should be run to confirm that at least 85% of the plasmid is in a covalently closed, circular conformation and that there are no signs of degradation. DNA should be resuspended at a high concentration (preferably > 5 mg/mL) to avoid diluting the electroporation buffer after mixing DNA with the cells.
Cell Culture and Handling
The most critical factor for ensuring efficient, reproducible transfection is working with low passage, healthy cells in log phase growth. Cells should be split one day before transfection, and adherent cells should be subconfluent when harvested for electroporation; freshly thawed and late passage cells generally do not transfect efficiently. Cell clumping can reduce transfection efficiency, so complete dissociation during pre-electroporation trypsinization and washing steps is essential.
Post electroporation, cells should be removed from the processing assembly immediately and rested at 37°C for 20 minutes before adding media or supplements. Allowing the cells to spread out in a multiwell plate or empty culture vessel during the resting period may increase oxygen transfer and improve cell viability. MaxCyte processing assemblies have been designed to maximize cell recovery; rinsing out processing assemblies to collect more cells is not advisable.
DNA Titration
Cell viability is inversely correlated with the quantity of DNA loaded into the cell due to DNA toxicity. Figure 1 illustrates how increasing concentrations of plasmid DNA lead to higher transgene expression but lower viability in CHO K1 cells. When developing an assay with a new cell type or loading agent, users should perform small-scale electroporations to identify a DNA concentration that yields optimal assay responsiveness and cell viability levels. Once optimized, conditions can be scaled up to transfect up to 2x1010 cells via Flow ElectroporationTM. Since some target proteins can reduce cell viability when expressed at high levels, DNA concentration optimization should be performed with the gene of interest in parallel with a reporter plasmid and with no DNA to determine the relative effects of electroporation, DNA toxicity and target toxicity on cell viability.
Figure1.
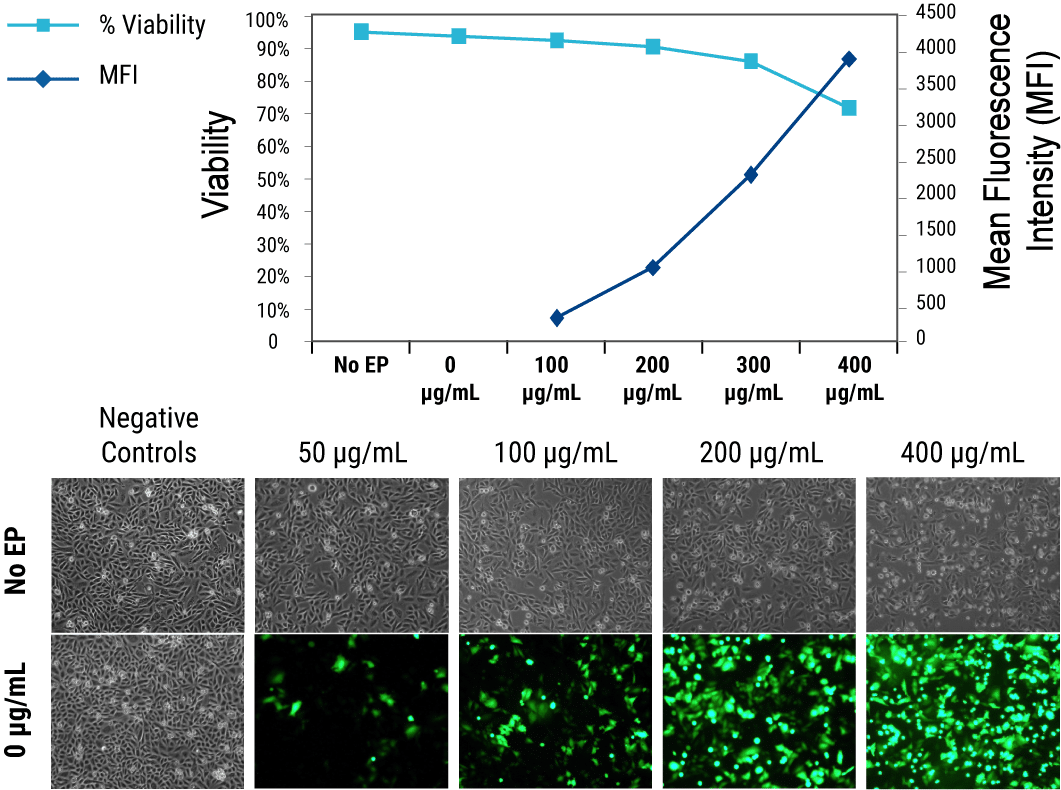
Figure 1. Inverse correlation between transgene expression and cell viability in transiently transfected CHO K1 cells. Cells were transfected with increasing concentrations of GFP expression plasmid DNA analyzed by FACS at 24 hours post electroporation. Viability was assessed by propidium iodide exclusion.
Plating and Analysis
Assay responsiveness varies between different cell plating conditions and the time between transfection and analysis; thus, it is advisable to optimize these parameters. The high cell viability following MaxCyte electroporation enables transfected cells to be seeded at varying densities in different plate formats. Cells should be assayed at several different time points after electroporation. In some cases, assays can be performed within 6 hours after transfection, while other assays may require multiple days post electroporation to reach peak sensitivity. A particular case to note is that some researchers screening ion channels report that culturing transfected cells at 28°C leads to higher cell surface expression levels, which may be beneficial in automated planar patch clamp assays.
Cryopreservation
MaxCyte® electroporation can be used to generate Assay Ready Cell banks by transfecting cells in bulk, then cryopreserving aliquots for future use. Different cell types, targets and assay applications require different cryopreservation regimens. In some cases, cells can be cryopreserved immediately after the post electroporation resting period; in other cases, cells must be plated or cultured before freezing. We recommended that users test several conditions for each new cell type, target or application. Figure 2 shows a typical experimental plan for optimizing cryopreservation conditions.
Figure 2.
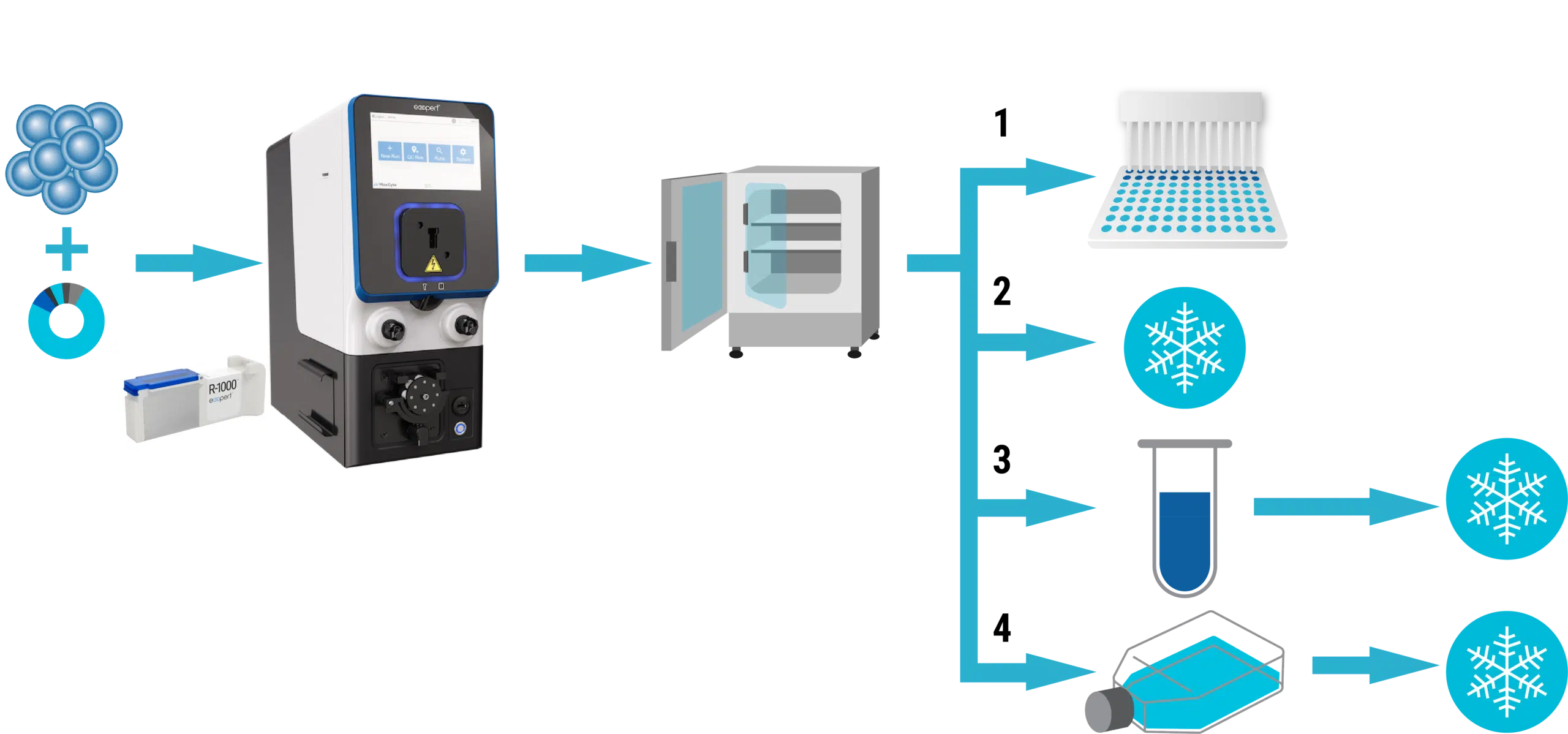
Figure 2. Workflow overview for optimizing cryopreservation conditions. After electroporation in an OC-400 or R-1000 processing assembly, cells are rested at 37°C for 20 minutes, then divided into four treatment groups: 1) plate without freezing and assay at 2-3 time points after transfection; 2) freeze immediately 3) incubate in medium for 1-2 hours before freezing; and 4) plate 18-48 hours before freezing.
Assay Ready Cells are typically frozen in 10% DMSO plus serum (20-90% v/v) or in commercially available cryopreservation solutions. Most users cryopreserve cells in ethanol-jacketed polycarbonate containers that are placed at -80°C for 18-48 hours before long-term storage in liquid nitrogen.
Summary
- High-quality DNA and healthy cells are critical for achieving optimal results with the MaxCyte® ExPERT™ electroporation platform.
- To minimize the effects of DNA toxicity and maximize assay performance, small-scale, static electroporation can be used to define transfection conditions which can be applied to Flow Electroporation® to transfect up to 2x1010 cells.
- A systematic approach to optimizing cell handling, analysis and cryopreservation conditions enables researchers to achieve the best possible results with their Assay Ready Cells.





