With a core focus on cell and gene therapy translation, the annual meeting of the International Society for Cell and Gene Therapy takes an integrative approach across all stakeholders to address key topics spanning translational research and preclinical development through to clinical trials, regulatory approvals, commercialization and, ultimately, patient access. Topics are curated to address the biggest bottlenecks in cell and gene therapy development.
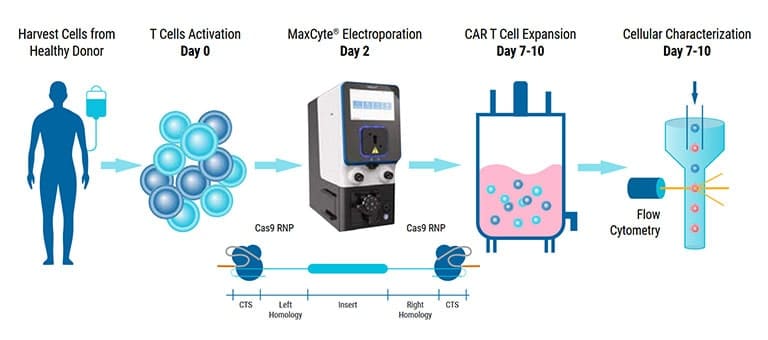
MaxCyte will showcase our cGMP-compliant nonviral cell engineering platform and how we can accelerate therapeutic development from concept to clinic utility.
Featured Presentation
Gene editing in hematopoietic stem cells for monogenic blood cell diseases
Friday, May 31, 2024, from 12:45 to 1:45 pm PT in Room 211
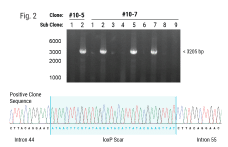
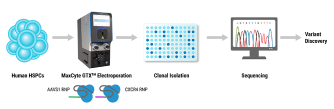
Hematopoietic stem cells (HSCs) are responsible for life-long production of all blood cells and can be collected, manipulated ex vivo for gene addition or gene editing, and re-engrafted by intravenous infusion. Gene therapy using lentiviral vectors has successfully achieved clinical improvements for more than a dozen hematological disorders, including primary immune deficiencies (PID), hemoglobinopathies, lysosomal storage and leukodystrophy disorders. While the safety profile of lentiviral vectors in HSCs has been good, lentiviral vectors lack precision in integration and may not recapitulate precise expression patterns needed for physiologically regulated genes. Gene editing of endogenous loci has the potential for precise insertion and physiologically regulated expression. A collaboration between investigators at UC Berkeley, UC San Francisco and UC Los Angeles has led to a clinical trial that will use Cas9-mediated homology-directed repair with a single-stranded oligonucleotide donor to revert the sickle cell disease (SCD)-causing mutation in HBB. Preclinical, investigational-new-drug-enabling activities involved translation of methods for gene editing in SCD patient HSCs to good-manufacturing-practices-compatible processes with subsequent cryopreservation formulation and release testing. Unlike SCD, where all patients have the identical single nucleotide mutation, most human genetic diseases have heterogeneous mutations across the affected gene in different patients. We are approaching two primary immune deficiencies (X-linked Hyper IgM Syndrome {X-HIM} and X-linked Agammaglobulinemia {XLA}) using Cas9-mediated site-specific insertion of minigene cDNA cassettes into the 5’ end of their respective genes (CD40L and BTK). Both of these genes require precise, physiological expression for safe and effective humoral immune reconstitution and the inserted normal copy of the gene will be under transcription control of the endogenous gene regulatory elements. The thresholds of engrafted gene-edited HSCs needed for therapeutic effects on antibody production are expected to be relatively low (5-20%), based on BMT studies in murine disease models of X-HIM and XLA. We have demonstrated restoration of normal expression patterns of each of these genes in patient-derived cells and in murine models, with development of appropriate B cell functions. We are applying adenine base editing to revert a recurrent stop codon in the CD3δ gene responsible for a genotype of severe combined immune deficiency (SCID) in an ethnic population. CD3δ SCID patient bone marrow CD34+ HSPC were treated with the adenine base editor to correct the CD3D mutation and had restored capacity to produce T lymphocytes in an in vitro artificial thymic organoid system. These advances in gene editing technologies are expanding the horizons for treating monogenic blood cell diseases.
Speakers during our presentation


Networking Event
Wrap the day with fellow researchers while meeting the MaxCyte team
Thursday, May 30, 2024, from 7:00 to 9:30 pm PT
The Butcher & Bullock Public House at 911 W Pender St, Vancouver, BC V6C 3B2, Canada
We are inviting all attendees of the 2024 ISCT annual meeting to an evening of networking. Join us at this exclusive event where you will have the opportunity to meet the MaxCyte team. Enjoy complimentary hors d’oeuvres and beverages while mingling with fellow scientists and researchers.
Registration for this event has now closed.
Oral and Poster Presentations
MaxCyte is excited to highlight researchers presenting at ISCT using our technology. Attend the oral presentations or stop by the posters below to see how scientists are using MaxCyte's technology in their work.
B7-H3 CAR T-cells manufactured via CRISPR knock-in at clinical scale are effective against small-cell lung cancer

Poster 956 presented by Vimal Keerthi, Clinical Process Development and Manufacturing Scientist at the Laboratory for Cell and Gene Medicine, Stanford University School of Medicine
Poster presentation: Wednesday, May 29, 2024, from 7:00 to 8:30 pm PT
Development of a GMP manufacturing process for base edited hematopoietic stem and progenitor cells (BE-HSPCs) for X-linked chronic granulomatous disease (X-CGD)
Poster 40 presented by Hong Lei
Poster presentation: Wednesday, May 29, 2024, from 7:00 to 8:30 pm PT
Oral presentation: Friday, May 31, 2024, from 3:45 to 4:45 pm PT