Strategies to accelerate product development and de-risk cell therapy manufacturing
Leverage MaxCyte’s clinically validated platform and extensive expertise to minimize risks
in your cell therapy programs
Cell therapy is rapidly advancing with increasingly complex cell engineering strategies. However, this complexity poses challenges in the manufacturing process, increasing the risk of failures. To mitigate these risks, selecting best-in-class manufacturing technology and clinically proven platforms is crucial.
MaxCyte’s clinically validated electroporation platform is designed to address key risk factors in cell therapy manufacturing. It successfully handles challenges related to process variability, product yield, and platform ease-of-use, enabling a reproducible and robust manufacturing process.
Additionally, our comprehensive technical and regulatory support includes expert teams that help navigate potential technical or regulatory hurdles. This additional support layer minimizes risks and boosts the overall success of your cell therapy initiatives.

1. Choose a clinically validated electroporation platform
The ExPERT™ GTx™ Electroporation System has been successfully enabling cell therapy clinical studies over the years with seamless scalability from early research through commercialization.
- Robust transfection process enables consistent and reproducible results across various donors and manufacturing sites
- Small footprint minimizes space requirement in the manufacturing suite
- Simplicity of instrument and processing assembly set up enable seamless tech transfer from process development to manufacturing and minimizes risk of operator-related errors
- Multiple pre-optimized protocols for over 80 cell types minimize time spent in optimization of electroporation parameters
- Fast processing time (8 mL per minute) minimizes impact in cell health
- Ability to process highly concentrated cells (0.5 to 2x108 cells/mL) enables multiple autologous and allogeneic applications
- Processing assembly options to process sample volumes ranging from 15uL to 100mL ensures seamless scalability
MaxCyte’s electroporation technology has been shown to minimize donor-to-donor variability.
Donor variability poses risks in primary cell therapy manufacturing, potentially leading to markedly different quality profiles due to individual cell responses.
Using MaxCyte’s technology, Richard Child’s group at the NIH achieved an average of approximately 90% knockout of CD38 across 8 different primary NK cell donors with a standard deviation of under 5% (Figure 1). Furthermore, NK cell viability, expansion, and functionality post-electroporation all had similarly small variances based on donor.
Minimal donor-to-donor variability helps ensure predictable outcomes, reducing risk of negative impact on the manufacturing process.
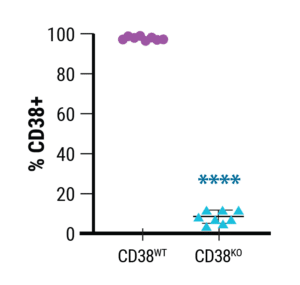
Figure 1. Relative expression of CD38 in wild-type (CD38WT) and CD38KO NK cells.
2. Maximize cell recovery to help achieve therapeutic dose
The newly developed R-20K™ and G-20K™ Flow Electroporation® Processing Assemblies are meticulously engineered to deliver high cell viability and exceptional cell recovery. These cutting-edge PAs facilitate rapid cell expansion, making them ideal for therapeutic development initiatives that rely on precious patient samples ranging from 5 mL to 20 mL in volume.
- Maximum cell recovery at all volumes, minimizing loss of precious cells
- Rapid sample processing in 1 – 5 minutes, minimizing impact to cell health
- Simple setup and ease of use in process development labs and manufacturing suite
- Sterile-weld adaptable for closed-process applications
- High energy protocols maintain cell viabilities for rapid expansion
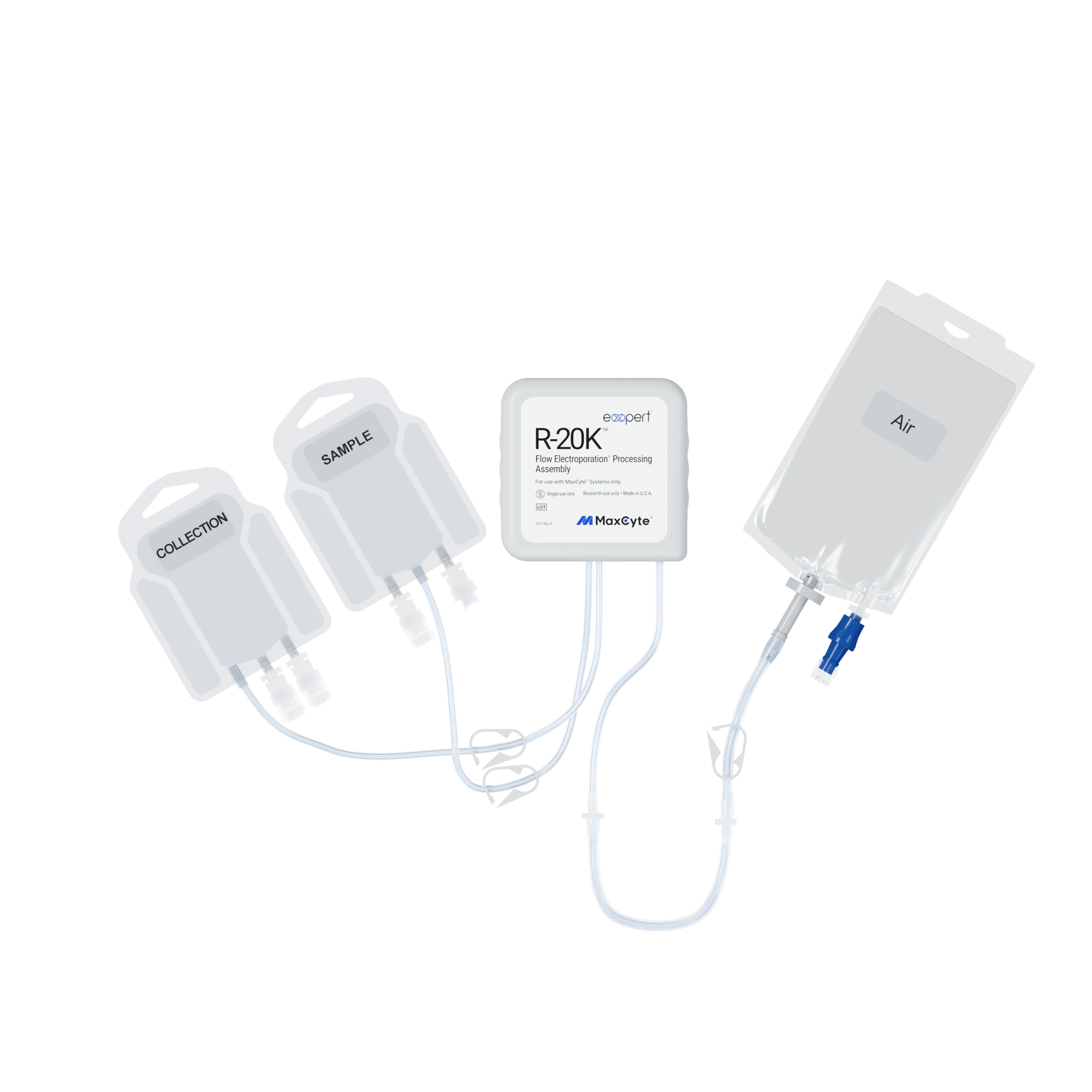
The R-20K™ Flow Electroporation ® Processing Assembly is designed for Research Use Only, while the G-20K™ Flow Electroporation® Processing Assembly is manufactured under cGMP and compatible with clinical applications.
3. Leverage over 20 years of expertise to help you navigate technical and regulatory hurdles
With over two decades of dedicated work, MaxCyte is the pioneer in applying electroporation to cell therapy. We have been at the forefront of advancing and refining our technology to meet the increasingly complex needs of cellular therapeutics.

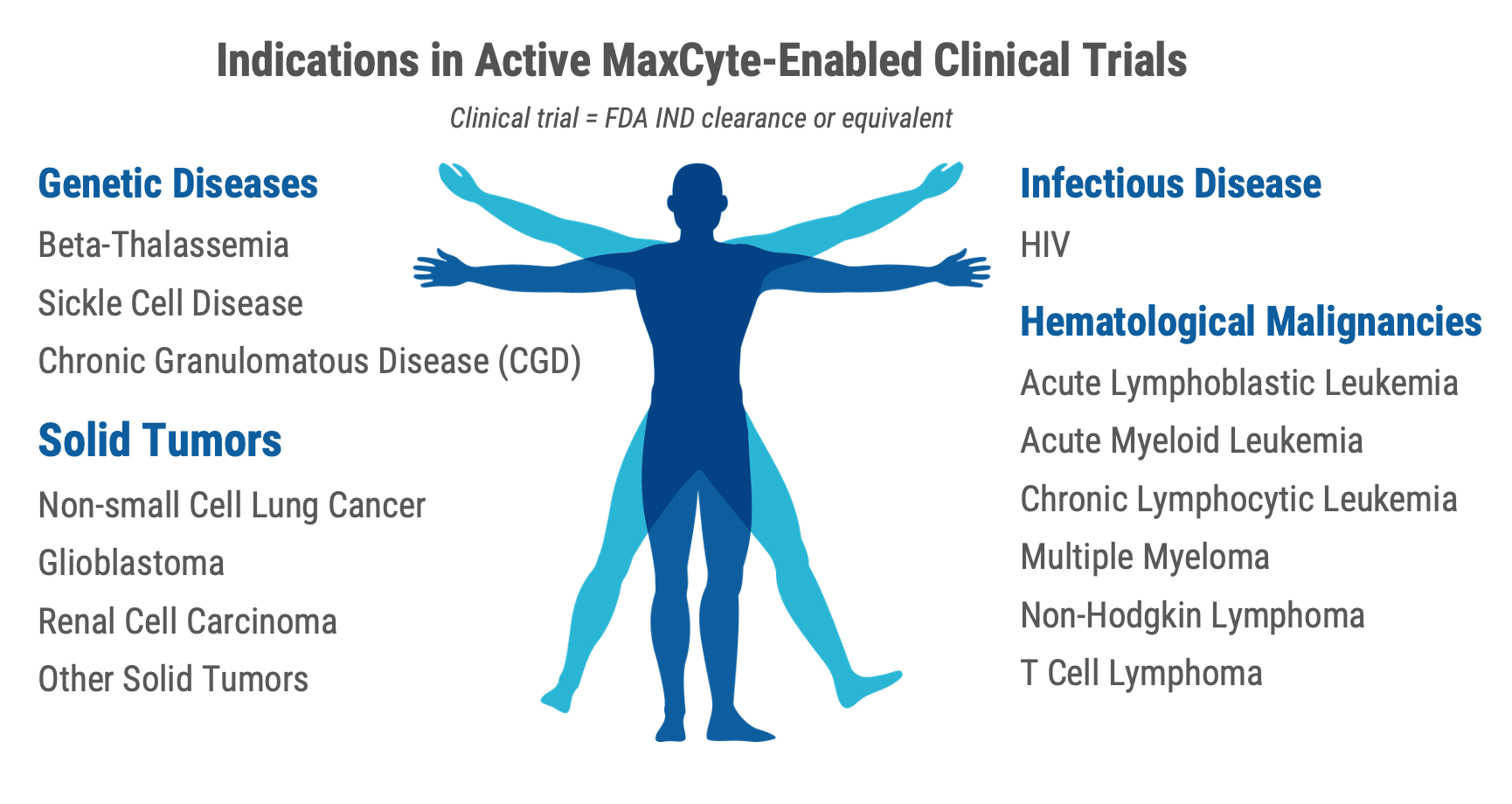
Together with our partners, we have enabled clinical trials across an expanding range of indications
Our best-in-class platform, unmatched scientific expertise and experienced regulatory support has been enabling our customers within a wide range of clinical applications.
1,000+
Estimated patients in active clinical trials enabled by MaxCyte
Our dedicated global scientific support team provides comprehensive assistance to our customers through the entire process, from optimization to tech transfer and manufacturing. Leveraging our extensive technical expertise, we strive to help accelerate the progress of cell therapy programs.
We have established partnerships with leading biotech companies, collaborating with 25 partners spanning various therapeutic areas.
Allogene
therapeutics
Beam
therapeutics
Caribou
Biosciences
CATAMARAN
BIO
CRISPR
therapeutics
CELULARITY
CURAMYS
EDITAS
MEDICINE
INVIOS
INNOVATIVE IMMUNO-ONCOLOGY
INTIMA
BIOSCIENCES
KITE
A GILEAD COMPANY
KSQ
LG CHEM
LYELL
IMMUNOPHARMA
MYELOID
THERAPEUTICS, INC.
NKARTA
THERAPEUTICS
PRECISION
BIOSCIENCES
SANA
BIOTECHNOLOGY
VERTEX
VOR
BIOPHARMA
WALKING FISH
THERAPEUTICS
VITTORIA
BIOTHERAPEUTICS
PRIME
MEDICINE
WUGEN
Regulatory Support
MaxCyte’s experienced regulatory team is well-equipped to assist our customers as they navigate approval processes. Our US FDA master file has been continuously updated since its initial filing in 2002 and has been referenced in 35+ INDs submissions.





