Scientific Brief
MaxCyte® Electroporation Enabled Immunocytokine Development
Abstract
Immunotherapy is a cutting-edge approach to cancer treatment that directs a patient’s immune system to target and kill tumor cells. Antibody therapies have been successfully used to treat lymphomas but have shown limited success against solid tumors, partly due to their lack of inherent anti-tumor activity. Immunocytokines, novel fusion proteins combining antibody specificity with the immune-stimulating activity of cytokines, may overcome this limitation. CEACAM-5 (CEA) is a promising target for immunocytokine (ICK) development due to high levels of expression on breast tumors. In this study, MaxCyte® electroporation enabled high-yield transient expression of an anti-CEA-IL-2 ICK that showed anti-tumor activity in a humanized mouse CEA+ breast cancer model.
Experimental Design
Anti-CEA-IL-2 ICK, or the parental anti-CEA antibody (M5A), was expressed in CHO-S cells electroporated with heavy and light chain expression plasmids in a 3:1 ratio using the MaxCyte STXTM. Purified protein was tested for IL-2 activity and for anti-tumor efficacy in a humanized CEA+ breast cancer mouse model.
Results
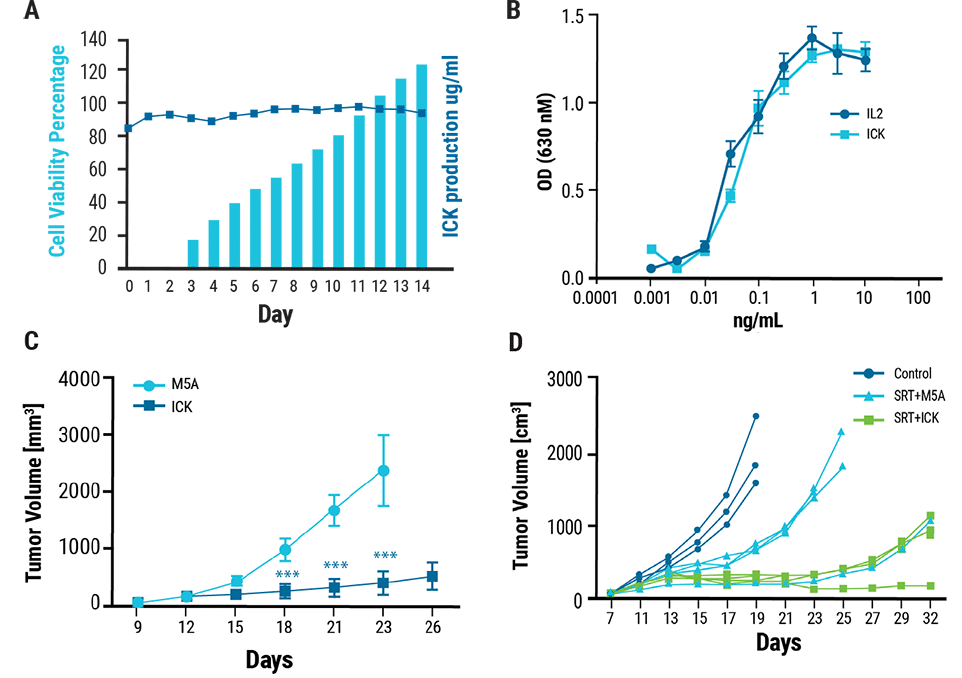
A) Transfected cells produced 120 μg/mL of ICK and high cell viability was maintained throughout the 14 day culture. Cytokine activity was measured using a HEK-BlueTM IL-2 reporter cell line. B) The activity of the ICK was comparable to that of recombinant human IL-2. A murine model of CEA+ breast cancer was established by injecting tumor cells into CEA transgenic mice. C) Following treatment with five daily injections of ICK or M5A (control), ICK-treated mice developed significantly smaller tumors over time compared to control mice. As radiation treatment is a standard cancer therapeutic, a radiation plus ICK combination therapy was tested in the murine CEA+ breast cancer model. D) Mice were treated with stereotactic radiation followed by four daily injections of ICK (SRT+ICK) or M5A antibody (SRT+M5A). Combination therapy with SRT+ICK significantly reduced tumor growth compared to treatment with SRT +M5A.
Summary
- MaxCyte enabled the transient expression of biologically active immunocytokine
- Transiently expressed ICK retains cytokine activity comparable to recombinant human IL-2
- Treatment with transiently expressed ICK inhibited tumor growth in a humanized mouse breast cancer model both as a mono-therapy and as a combination therapy with SRT
- MaxCyte is an essential technology platform for high-yield transient expression of complex biologics, enabling fundamental research and novel therapeutic development
References
- Kujawski M, Sherman M, Hui S, et al. Potent immunomodulatory effects of an anti-CEA-IL-2 immunocytokine on tumor therapy and effects of stereotactic radiation. Oncoimmunology. 2020;9(1):1724052. doi:10.1080/2162402X.2020.1724052





