Case Study
From Roadblocks to Breakthroughs, Unlocking Clinic-Ready Efficiency
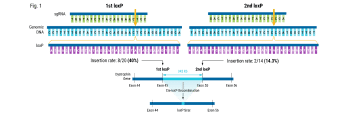
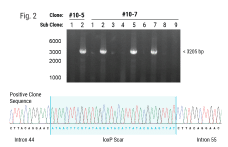
In this case study, Andrew shares a collaboration story about how we were able to help an established cell therapy developer who was struggling to achieve the necessary efficiencies needed to progress their product into the clinic. Through teamwork between the MaxCyte Field Application Scientist team and the developer's scientific team, they identified modifications to the knockout strategy, resulting in a significant efficiency boost from 15-20% to 90%.
This breakthrough allowed the developer to progress from a state of uncertainty to confidently initiating the IND process and proceeding to clinical manufacturing. The success underscores the importance of not only technological innovation, but also collaborative relationships in accelerating the delivery of life-saving therapies to patients.
Senior Field Application Scientist

Leverage MaxCyte’s pioneering expertise in cell therapy to accelerate product development and de-risk manufacturing
Employing our clinically-validated electroporation platform you can expedite process optimization and seamlessly transition to the clean room.
Our meticulously designed consumables maximize cell recovery to help achieve therapeutic dose.
With partnerships established with 25 leading biotech companies, our unmatched scientific and regulatory support help you navigate technical and regulatory hurdles.

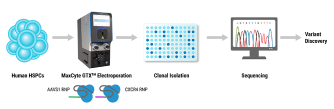
Understanding Flow Electroporation
Electroporation is a non-viral transfection technique that uses electricity to relax cell membranes, allowing payload to enter. Our Flow Electroporation technology is uniquely designed to allow cells to flow through the processing chamber where discrete volumes are electroporated, then collected on a continual basis. This pioneering innovation makes genetic engineering at large scale a reality.