Let's Build Better Cells Together
Navigate your way from concept to clinic by partnering with MaxCyte®.
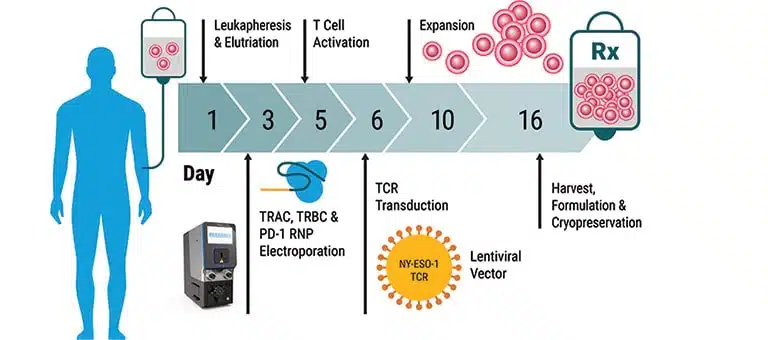
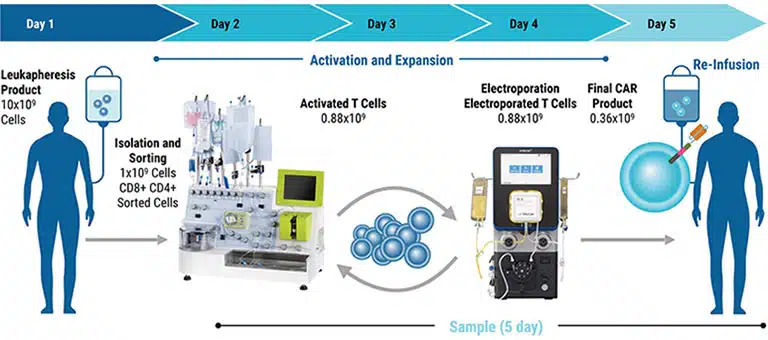
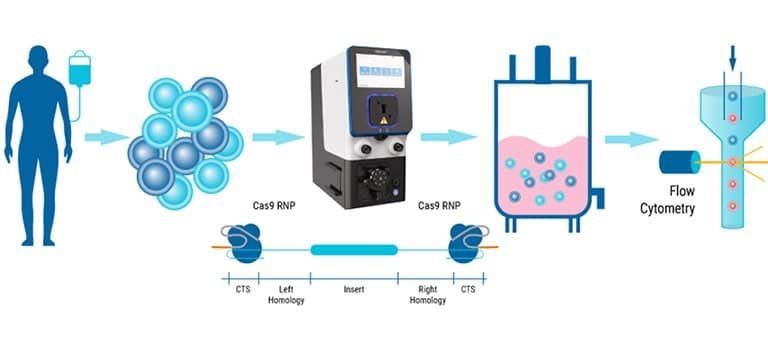
We've spent over 20 years perfecting the art of cell engineering and venturing beyond today's process to innovate tomorrow's solutions. Our ExPERT™ instruments feature best-in-class electroporation technology combining high efficiency and cell viability with seamless scalability, all backed by a global team of technical and regulatory experts providing ongoing support.
We provide the spark of collaboration empowering research into treatments and cures that will transform human health. Chart your best course with MaxCyte.
Explore the research we've supported
See how MaxCyte technology is advancing our partners' biomedical innovations