Poster
Electroporation of a Non-Integrative DNA Nanovector for Efficient, Semi-Automated, GMP Manufacturing of CAR T Cell Therapies
Abstract
Recombinant T cell expression of Chimeric Antigen Receptors (CARs) has shown extraordinary efficacy in numerous clinical trials as an adoptive cell therapy to treat hematological malignancies. Still, CAR T therapy faces significant challenges, ranging from long lead times and expensive manufacturing to complicated vector-engineering. Also, CAR T cell production routinely employs random integration of viruses or transposons, which carries an inherent risk of genotoxicity and costly, long-term patient follow-up. CAR engineering by transient mRNA transfection could be safer but more cost-prohibitive, requiring several doses per patient. DNA is a promising alternative but can cause sensitive T cells to lose functional capacity or induce apoptosis.
Here we describe Nano-S/MARt (nS/MARt), a novel DNA vector platform for stable CAR expression with minimal disruption of T cell activity. This antibiotic- free, nanovector technology uses scaffold/matrix attachment regions (S/MARs) for DNA vector maintenance and replication and transfects primary human T cells efficiently and without toxicity. When combined with GMP-compliant MaxCyte Flow Electroporation® and CliniMACS Prodigy® automated cell processing, nS/MARt enabled the production of recombinant T cells with stable CAR expression and enhanced anti-tumor activity in only five days. The result was a shortened manufacturing protocol, producing safer cell therapeutics for thousands of patients from a single batch.
Optimized Nanovectors Provide Prolonged Transgene Expression in Primary CD3+ Cells
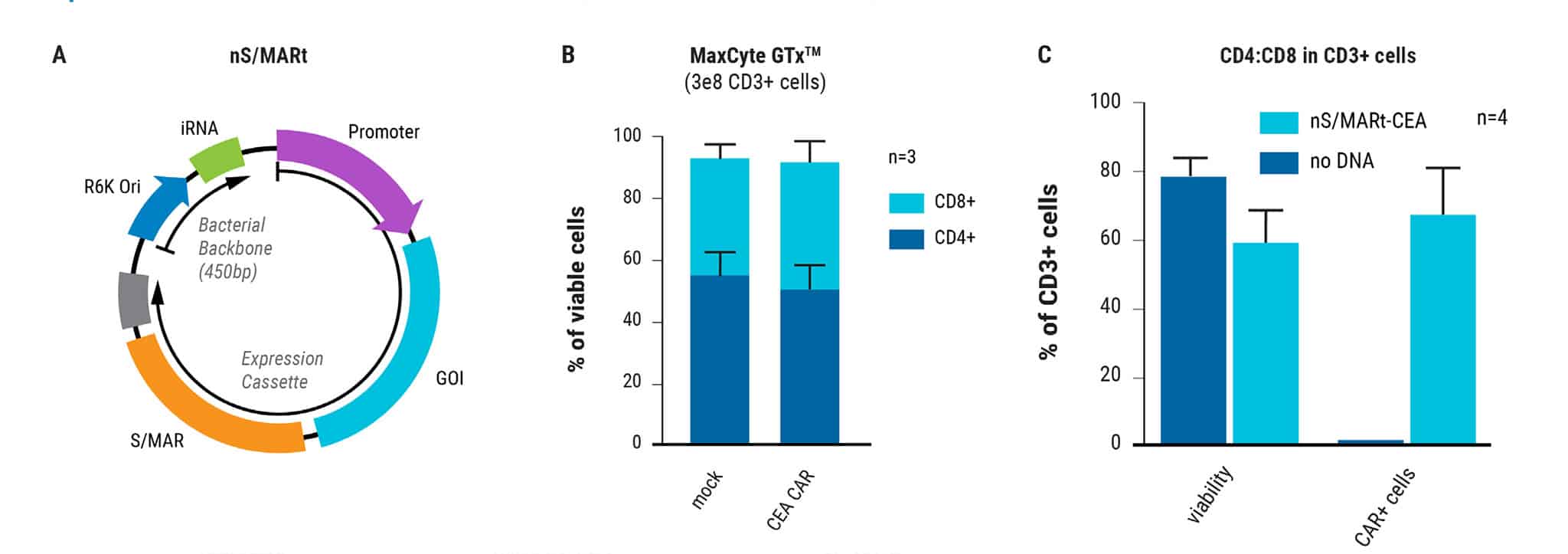
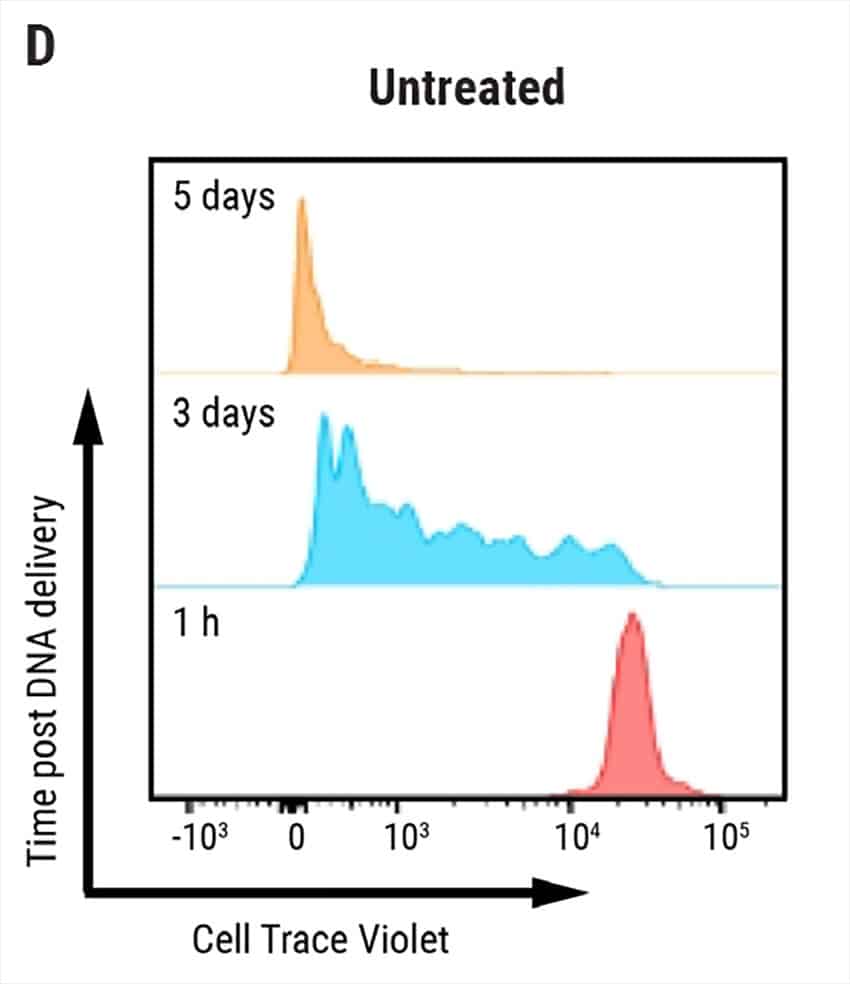
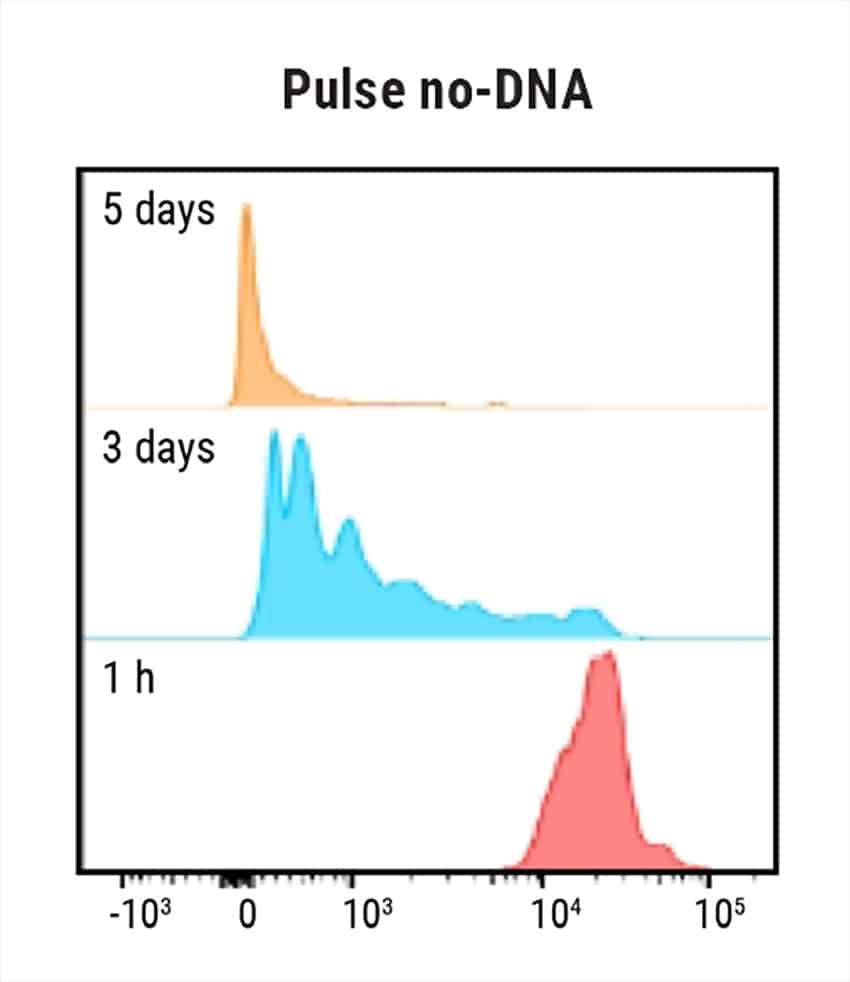
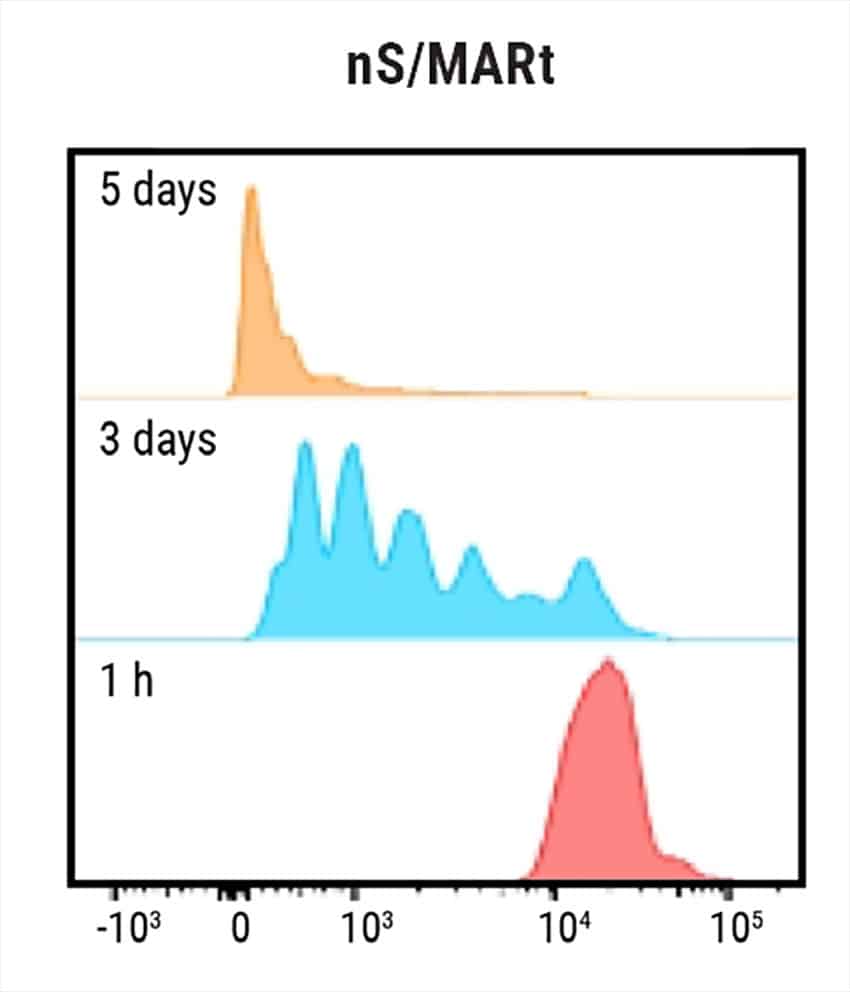
Figure 1: Electroporation of nS/MARt vectors provides long-term transgene expression in T cells. A) Schematic of a generic nS/MARt vector. B) nS/MARt vector expressing the CEA-CAR was delivered with high efficacy and viability into activated human CD3+ cells with the MaxCyte ExPERT GTx® instrument; 125 μg/ml of DNA was delivered to 3 x 108 cells using a CL-1.1 Processing Assembly and the Expanded T cell 3 program. C) Upon transfection with nS/MARt plasmids, the CD4:CD8 ratio in cells of healthy volunteers was not affected (n=3), and D) the proliferation of CD3+ cells treated with nS/MARt vectors was not affected.
nS/MARt Delivery by Electroporation Has Minimal Impact on Human T Cells and Provides Superior Functionality
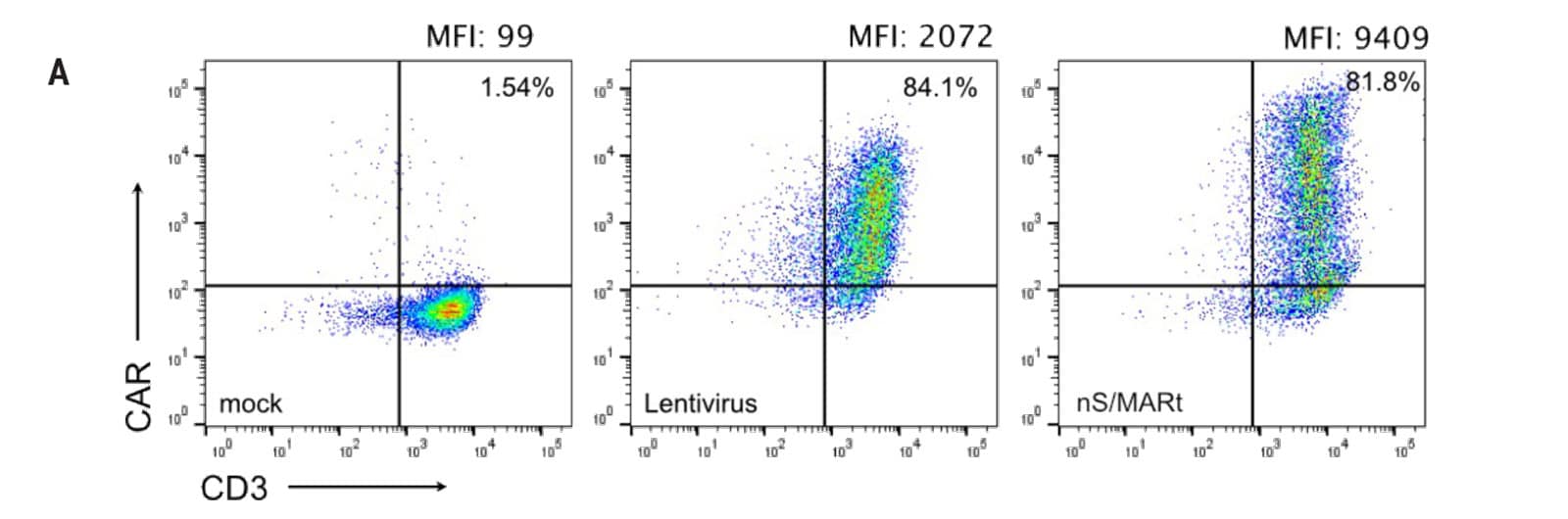
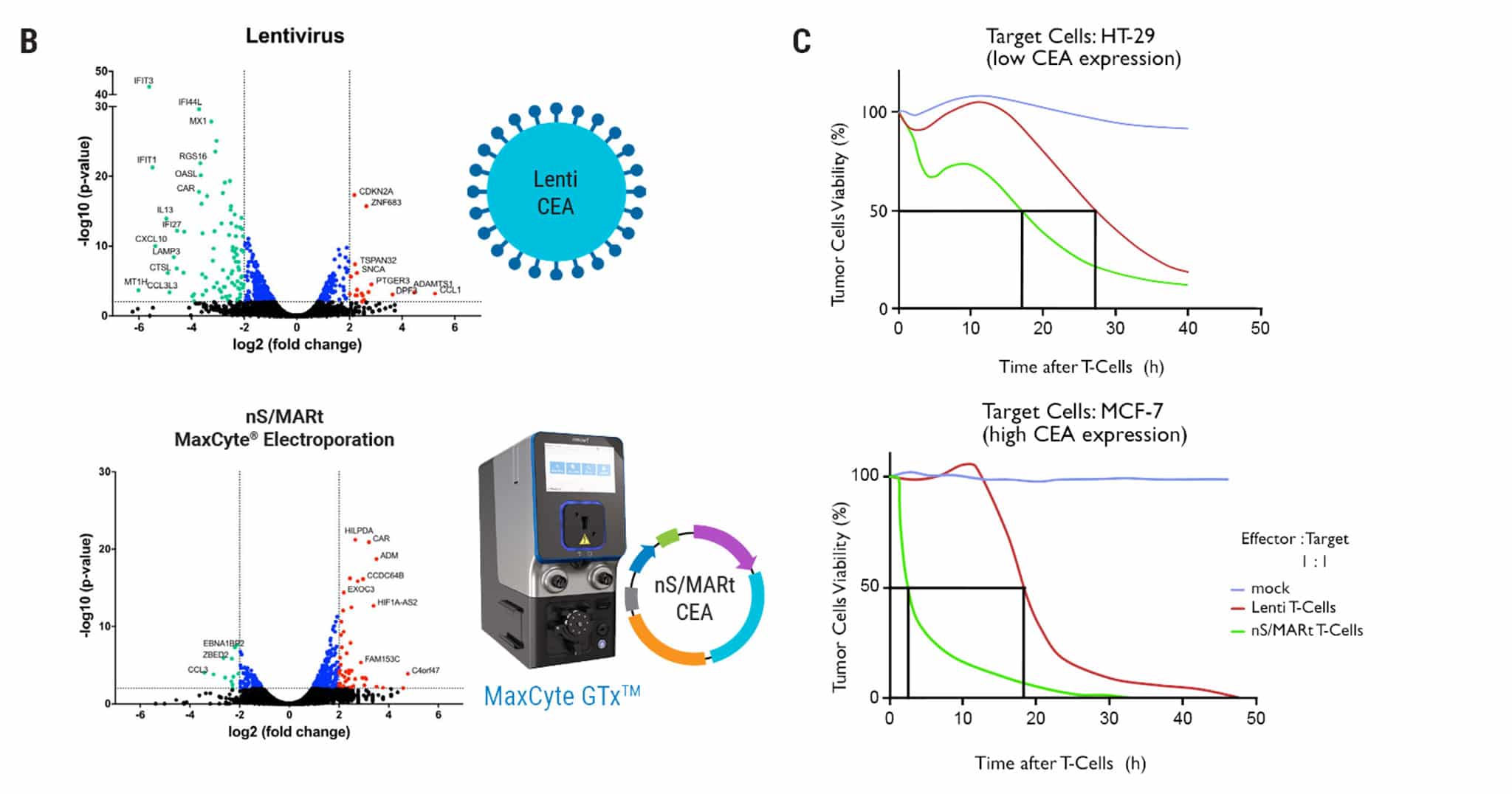
Figure 2: Transcriptome and functional analysis of nS/MARt CAR T cells. A) CEA-CAR T cells generated by MaxCyte® electroporation with nS/MARt or by lentiviral transduction were analyzed by FACS for expression of the transgene and compared to mock cells. Both methods produced a similar percentage of CAR T cells. B) Volcano plots display the transcriptional profile of lentivirally transduced CAR T cells, nS/MARt electroporated CAR T cells or mock electroporated T cells. When the transcriptional profile of T cells transduced with lentiviral vectors was compared to that of unmodified parental cells, 106 genes were differentially expressed. The most prominently regulated genes belonged to the IFIT family or encoded for chemokines. Interestingly, electroporation of nS/MARt led to only 61 differentially regulated genes. C) The efficacy of tumor target killing was assessed in a real-time in vitro killing assay, where 2.5 x 104 target cells were seeded on day 0, and effector CAR cells were added on day 1 at a 1:1 effector-to-target ratio. The EC50 was shorter for effector nS/MARt CAR T-generated cells than for cells generated with LV.
nS/MARt T Cells Mediate Efficient Tumor Killing in vivo
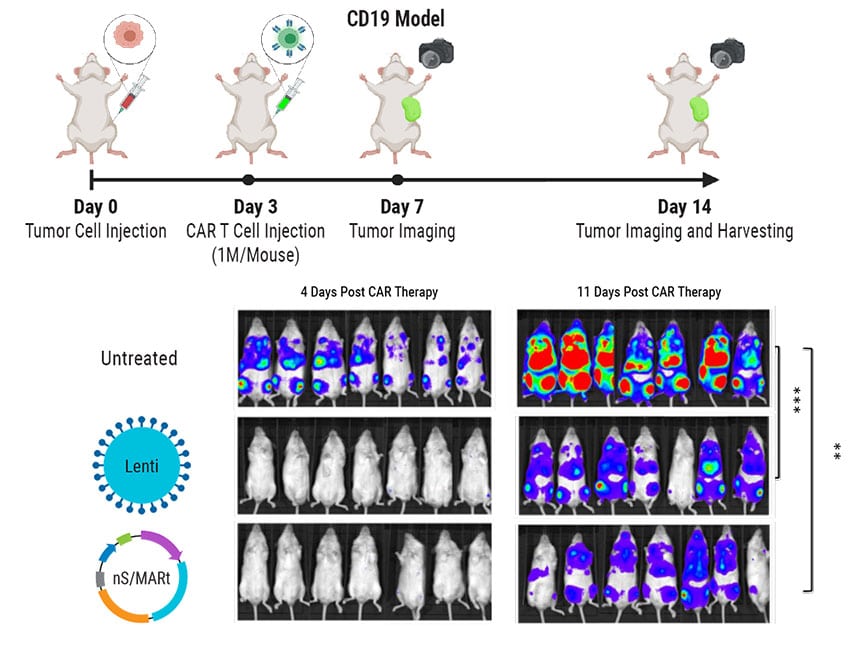
Development of GMP-Compatible, Large-Scale CAR T Cell Manufacturing with nS/MARt Vectors
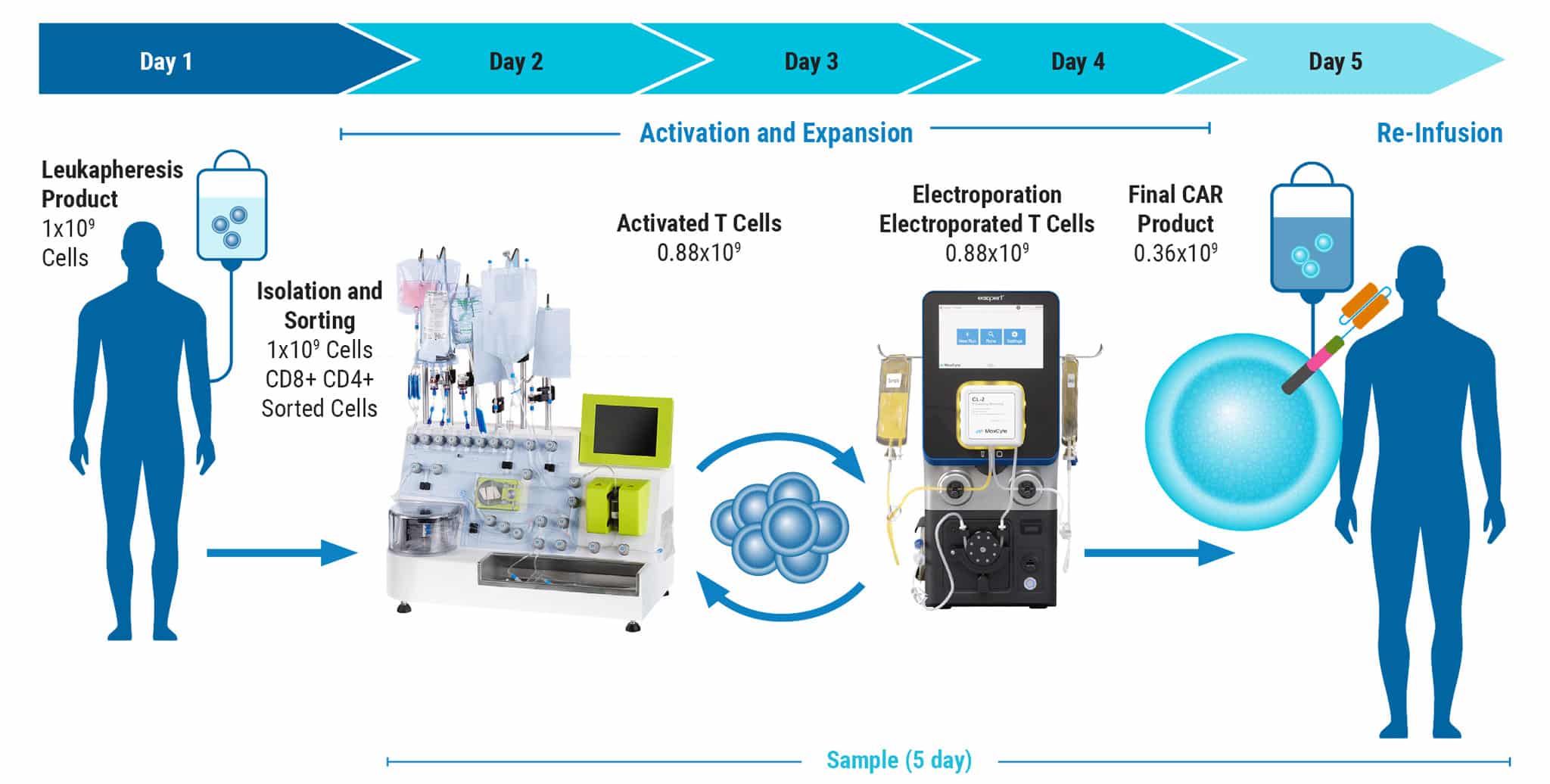

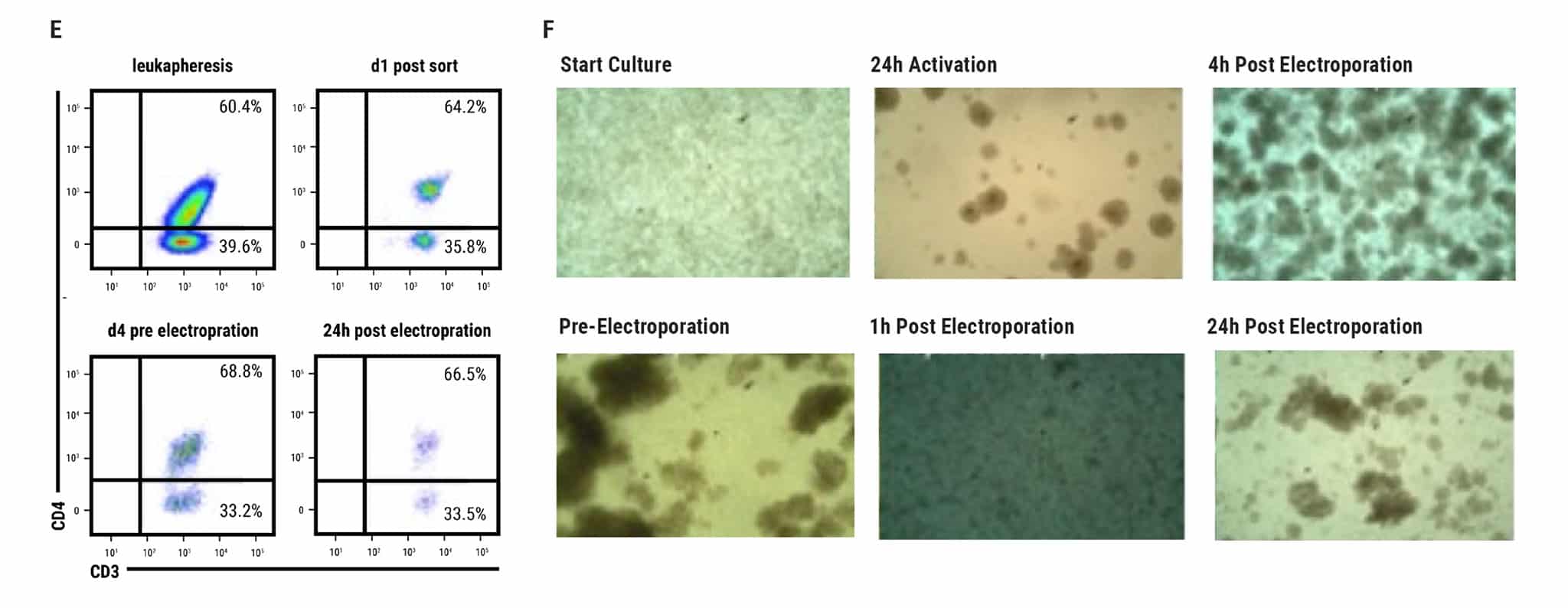
Figure 4 continued: Large-scale, GMP-compatible production of nS/MART T cells. For translation into a clinically relevant application of nS/MARt T cells, a manufacturing protocol to allow the generation of clinical-grade recombinant T cells was developed. For this, we coupled the CliniMACS Prodigy device (Miltenyi®), a fully automated and closed system for the isolation and culturing of primary human CD3+ cells, with the ExPERT GTx extra-large scale electroporation platform (MaxCyte®). A) CD3+ cells were isolated from a leukapheresis product with the TCT process using the CliniMACS Prodigy and activated for 3 days with T Cell TransActTM, IL-7, and IL-15. On day 3, 1 x 108 cells were counted and electroporated with 125 μg/mL of DNA using the GTxTM device. B) Shortly after electroporation, the cells were returned to the CliniMACS Prodigy and fed with IL-7- and IL-15-supplemented medium for one day. On day 5, cells were harvested and analyzed for CAR expression. C) The capability for killing tumor cells was tested in an in vitro killing assay D) and INF-γ production measured.
E) FACS plots show the same percentage of CD8 and CD4 cells within the CD45+/CD3+ subset before and after manufacturing. F) Samples taken from the CliniMACS Prodigy at different time points pre and post electroporation show the T cells’ proliferative capacity.
Summary
Enabling function of ExPERT GTx Flow Electroporation:
- Efficient and consistent cargo delivery with more than 80% of viable cells expressing reporter gene.
- Electroporation did not alter CD4:CD8 ratio (compared to mock electroporated cells).
- Electroporation did not impair T cell proliferation (compared to unpulsed CD3+ cells).
- Minimal disruption of transcriptomic profile, electroporated cells displayed a more naïve phenotype.
- More active and highly efficient killing capabilities of T cells.
- Large scale MaxCyte Flow Electroporation allowed to modify 3 x 108 cells yield 50-75% viability and 65-80% efficiency (donor dependent).
- MaxCyte electroporation proved to be gentle, and when combined with a highly optimized vector, resulted in cells that were more potent at eliminating tumors than standard LV-based therapies.





