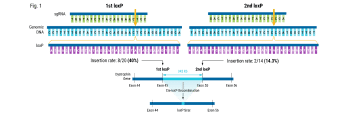
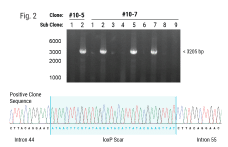
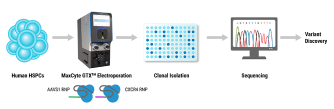
The American Society of Gene and Cell Therapy’s (ASGCT) Annual Meeting, is the premier event for professionals in gene and cell therapy. It is the best place for people in the field to learn from the latest scientific research, stay up to date on new technologies, and make career-advancing connections with peers.
Come visit us at booth #355 and discover MaxCyte’s cGMP-compliant non-viral cell engineering platform and how it enables the acceleration of therapeutic development from concept to clinic.
Watch our featured panel discussion:

Diversity of Therapeutic Approaches: Global Challenges, Opportunities, and Views from the United States, Europe, and Asia
Abstract
The session will feature three key opinion leaders from the United States, Europe and Asia who will discuss significant challenges, opportunities and views from their geographies. Topics covered during the session will include dialog about the diversity of cell types utilized, differing technological approaches and key regulatory & accessibility challenges they face during their journey to develop effective therapies.
Scientific Poster:
Modification of Conventional Mouse Dendritic Cells
Thursday May 18, 12PM -1PM
Abstract #1248
Poster Presenter: Shelby Namen, Washington University, School of Medicine
Abstract
Dendritic cells play an important role in bridging the innate and adaptive immune response. Conventional dendritic cells (DCs) are the most potent antigen presenting cell (APC) in vivo, but due to their relative scarcity and difficulty to culture, far less work has been done to optimize techniques for genetically modifying them relative to other myeloid cells, such as macrophages or monocyte-derived dendritic cells. Here we aimed to develop and refine permanent and transient methods to modify/engineer mouse conventional dendritic cells (DCs), so that they may be more effectively used in cancer immunotherapy and vaccine studies. We tested various viral transduction methods including three different adenoviral vectors (Ad5, AdRGD, and Ad5PK4) and a retroviral vector (SFG). In addition, we compared and tested electroporation with DNA and mRNA using the MaxCyte ExPERT GTx. Optimized electroporation with mRNA yielded the greatest transfection efficiency (~85%) of all methods and was able to be maintained for up to 9 days, while optimized retroviral transduction yielded moderate transduction efficiency (30-60%) with long-term expression. Electroporation with DNA was able to modify cDCs (8-15%) but yielded poor cell viability. Ad5 transduced cDC1s at a low rate of ~1%, while AdRGD improved transduction by 50-fold, and Ad5PK4 improved transduction by about 80-fold. These studies provide a variety of new methods for effectively engineering and/or modifying mouse conventional DCs.