MaxCyte Minutes Newsletter – Q4 2022
As the end of the year draws near, we’d like to take this opportunity to thank you for your support. At MaxCyte, we share your passion for enabling the discovery, development and manufacturing of next-generation medicines, harnessing the power of living cells to transform lives.
We are honored to support such a transformative industry, developing novel cell-based treatments that provide hope and new options to patients and their families worldwide.
All of us at MaxCyte, wish you a wonderful holiday season and happy new year!
Your MaxCyte Team
IN THE NEWS

MaxCyte Grand Opening Ceremony
MaxCyte® is thrilled to have moved into a new home within our Rockville, MD, community. On September 20th, we had the opportunity to stand alongside our friends and colleagues and cut the ribbon to our new headquarters.
We’d especially like to thank Michelle McMurry-Heath, President and CEO of the Biotechnology Innovation Organization (BIO), Secretary Mike Gill from the Maryland Department of Commerce, and Brad Stewart from the Montgomery County Economic Development Corporation for sharing a few words with us during the event and supporting this momentous occasion for MaxCyte and all our team members.

SPL News
We are happy to announce the signing of a new milestone partnership with Curamys. Curamys is a South Korean biotechnology company that develops cell & gene therapy using cell fusion technology to treat rare intractable diseases, including Duchenne muscular dystrophy and amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease).
MaxCyte is honored to support Curamys’ effort to develop its cell-fusion technology for novel cell-based treatments that provide hope and new options to patients and their families by using our Flow Electroporation® technology and ExPERT™ platform.
NEW PRODUCTS
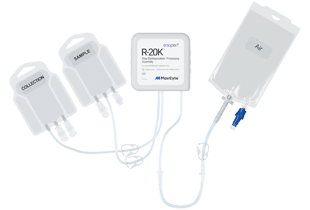
R-20K™ Processing Assembly
Maximized cell recovery for autologous cell therapy and biologics development.
Cells are precious, especially when developing autologous therapies and the small scale involved for these targeted treatments.
We are happy to introduce the new R-20K processing assembly for the MaxCyte ExPERTTM platform. The R-20K is specifically designed to ensure maximum cell recovery during small-scale development and production.
Used with the ExPERT STx® platform, the R-20K enables you to preserve precious cells and get the best out of your cell therapy or mid-scale biologics program.
CELL & GENE THERAPY
Webinar: Beyond affinity: antibody discovery, optimization, and developability screening from mammalian display libraries
In case you missed our webinar, it is now available on-demand. This insightful Labroots Webinar featuring Peter Slavny from IONTAS’ focuses on the development of a novel mammalian display technology for antibody discovery and learning how the system can be used to screen out candidates with poor developability profiles. Learn how using MaxCyte’s electroporation technology enabled the creation of these large mammalian display libraries.

Featured Presentation: Expanding the Boundaries of Cell & Gene Therapy
We had the honor to present at the BioInnovation Conference this year. This informative session featured frontier-leading speakers pushing boundaries to create a new generation of therapies that will enhance patient outcomes through cell engineering, RNA engineering and AAV gene therapy.
Moderator:
Lesley Eschinger, Director of Market Development, MaxCyte
Panelists:
Cenk Sumen, Chief Scientific Officer, MaxCyte
Dr. Milos Miljkovic, Chief Medical Officer, Cartesian Therapeutics
Craig Malzahn, Senior Vice President, Technical Operations, REGENXBIO

Pharma Voice Article: It’s electric — speeding up the cell therapy process for scalability
“What’s really unique about what we do is that we do it at a large scale so that you can get a therapeutic dose. We also do it with a highly efficient process so you get the most cells as you possibly can engineered.” MaxCyte President & CEO, Doug Doerfler, recently spoke with PharmaVoice about our Flow Electroporation™ technology and its applications, as well as cell therapy, CRISPR and more. Learn about the platform and the ways in which MaxCyte supports its customers.

BIOPROCESSING

Presentation: Accelerating Biotherapeutic Development Using a Scalable, High Performance transient transfection technology
Our Senior VP of Technical Applications, Dr. James Brady, presented at the BioProcess International on accelerating biotherapeutic development using our cutting-edge transient transfection technology. MaxCyte has developed a large-scale flow electroporation platform yielding gram quantities of proteins with desired post-translational modifications while maintaining high cell viabilities and transfection efficiencies. This is a rare opportunity to learn from one of the leading experts in the field, so be sure to download the presentation!

PHARMA ALMANAC ARTICLE
MaxCyte Chief Scientific Officer, Cenk Sumen, recently spoke with Pharma’s Almanac about industry inefficiencies and provided insight on transient expression and electroporation as a solution.
“It’s always best to fail fast and narrow your range of options quickly with confidence. Before specific candidates can be eliminated from consideration, developers must feel confident they can test them using multiple methods and assays — to fail not only fast but to fail confidently.”
FOCUS ON

Gene Zhu, VP of Regulatory
In this issue, we welcome Gene Zhu, our new Vice President of Regulatory. Learn more about his passion and vision for this critical role as we keep growing and supporting our partners through their development and commercialization process.
COME MEET WITH US

February 21, 2023 - February 23, 2023
London, UK

February 27, 2023 - March 1, 2023
San Diego, CA





