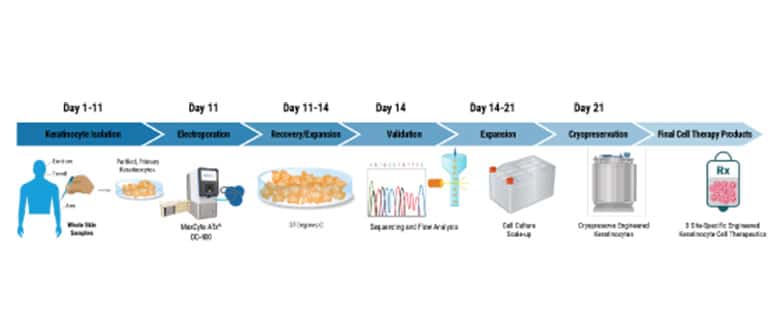
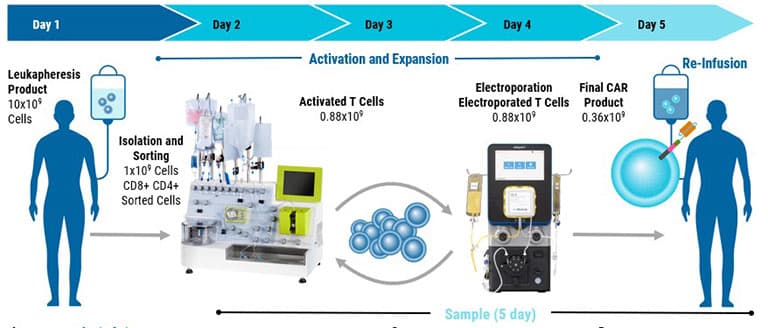
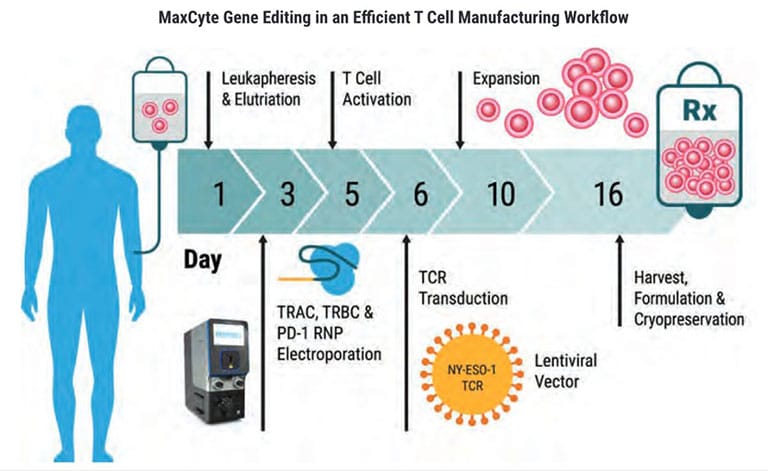
Cell Therapy Manufacturing
The advent of cell and gene therapies has ushered in a bold new era of medicine, one where previously incurable diseases now have treatment options.
As you work to bring the promise of these innovative therapies into the clinic, MaxCyte® is here to accelerate your journey to success.
Designed with cell and gene therapy in mind
Over 20 years ago, MaxCyte's technology was designed to overcome cell and gene therapy development barriers.
Today we partner with researchers who are actively assessing these therapies in clinical trials for treatment of genetic disease, hematologic malignancies, solid tumors and more. We are proud to be a part of many of the pioneering studies in this field.

Breaking technological barriers
One of the key challenges in cell therapy manufacturing is the need to produce large quantities of high-quality therapeutic cells efficiently. Traditional methods often fall short in terms of scalability and can be time-consuming. GTxTM overcomes these limitations by enabling rapid delivery of gene editing tools, at any scale, thus streamlining the manufacturing process.
- Reduce Manufacturing Time
- Reduce Batch-to-Batch Variability
- Enable Scale-up
- Reduce Cost
- Allow Flexibility in Process Design
How we enable cell and gene therapy development
Over the many years we've dedicated to optimizing and advancing electroporation, we've perfected an innovative technology that achieves key goals to streamline cell and gene therapy development:
- Highly efficient delivery of diverse payloads
- Nucleic acids
- Proteins
- Small molecules
- High cell viability after electroporation
- Seamless scalability with the same technology and general protocols from concept to clinic
- Simplified workflow with MaxCyte's Electroporation Buffer


Designed for compliance
Our GTx technology is designed with regulatory requirements in mind and supported by a team with over 20 years of experience. With a US FDA master file including all components of the system - instrument, buffer and processing assemblies. GTx helps manufacturers meet regulatory expectations and accelerate the development and commercialization of their products.
Leverage MaxCyte's pioneering expertise in cell therapy to accelerate product development and de-risk manufacturing
Employing our clinically-proven electroporation platform you can expedite process optimization and seamlessly transition to the clean room.
Our meticulously designed consumables maximize cell recovery to help achieve therapeutic dose.
With partnerships established with 26 leading biotech companies, our unmatched scientific and regulatory support help you navigate technical and regulatory hurdles.

Advance with confidence through gene editing outcome risk assessment
SeQure™ provides comprehensive gene editing outcome risk assessments, characterizing both on- and off-target activity alongside structural rearrangements. Our suite of proprietary, variant-aware assays delivers sensitive, biologically meaningful insights to support genome integrity, minimize risk and meet evolving regulatory expectations. Whether screening guides or preparing for IND submission, we help you move forward with clarity and confidence.
Research applications
Resources
Ready to learn how we can simplify your cell and gene therapy development?
Answers are just a click away!