MaxCyte's Partnership Model: Your Pathway to Development
MaxCyte empowers cell therapy developers with a proven partnership model tailored to your needs. From discovery to commercialization, we provide scalable technology, regulatory support, and unmatched cell engineering optimization.
Our non-viral cell engineering solutions accelerate development timelines and can drive cost savings of up to $12M. Trusted in 70+ clinical trials, and the only commercial-stage non-viral therapy, MaxCyte ensures scalability and compliance while mitigating risks.
Let us guide your development journey through every phase, from achieving early clinical success to global market entry.
Explore how MaxCyte can support your pathway to success.
MaxCyte Best in Class Technology
MaxCyte pushes the boundaries in cell therapy development with cutting-edge, non-viral engineering technology
The ExPERT™ GTx™ Electroporation Instrument has consistently supported clinical and commercial manufacturing with seamless scalability.
- Robust transfection process ensures consistent, reproducible results across diverse donors and manufacturing sites.
- Compact design minimizes space requirements in manufacturing suites.
- Simple instrument and processing assembly setup enable seamless tech transfer from process development to manufacturing, and minimizes operator-related errors.
- Multiple pre-optimized protocols for over 80 cell types minimize time spent in optimization of electroporation parameters.
- Fast processing time (8 mL per minute) minimizes impact in cell health.

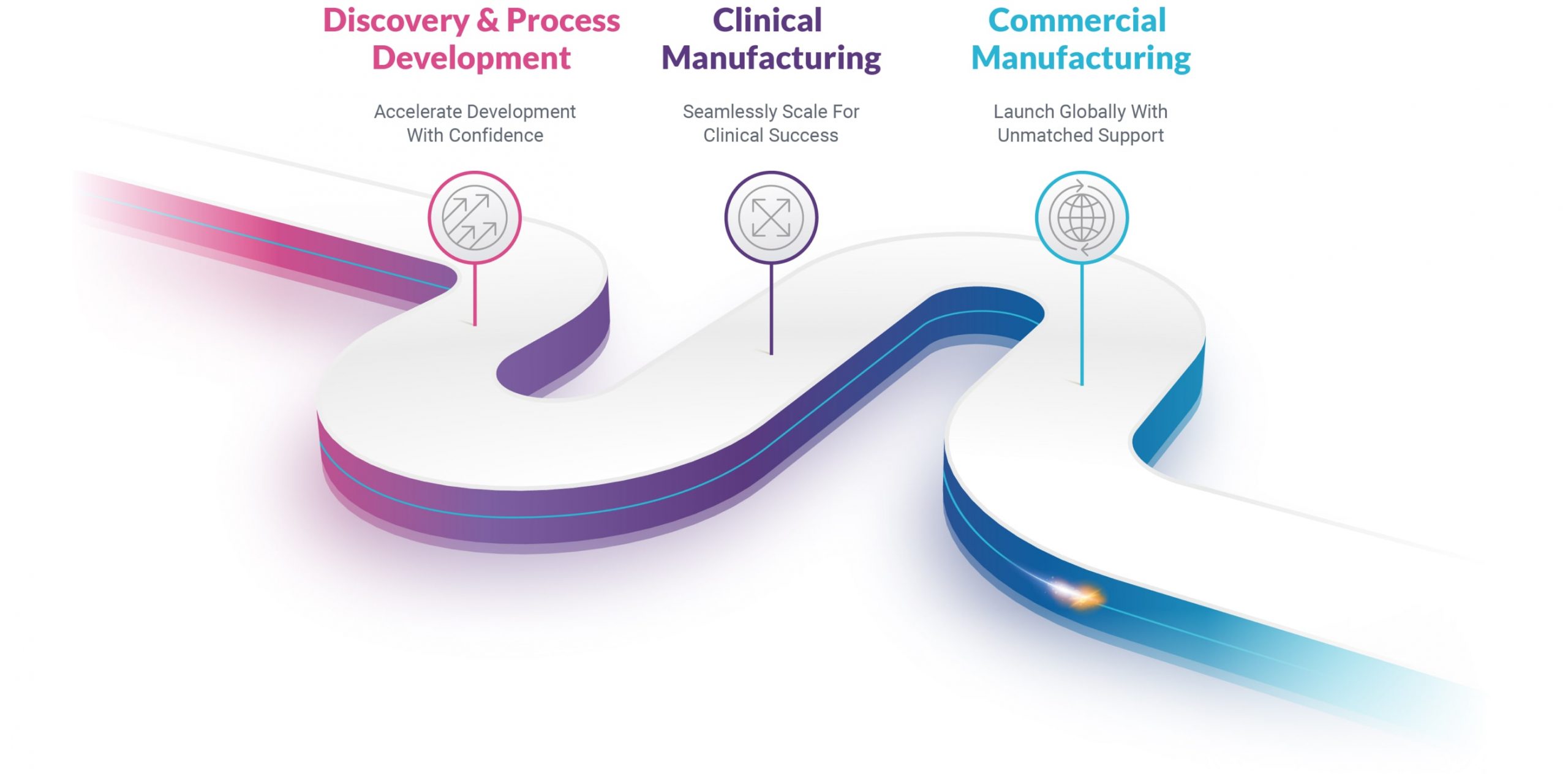
Your Pathway to Development
Discovery
Gain direct access to MaxCyte experts for on-site electroporation optimization and seamless scaling to advance your research.
Clinical Manufacturing
Receive seamless tech transfer support ensuring real-time solutions for clinical production challenges.
Commercial
Benefit from lifetime support with 24/7 assistance, quality assurance, regulatory support, and on-demand MaxCyte insights for production success.
Your License, Your Advantage
A strategic platform license underpins our partnership model, empowering your pathway to cell and gene therapy development. With tailored flexibility, regulatory expertise, and cutting-edge technology, the license ensures access to our robust IP and know-how, enabling a seamless progression from clinical development to commercialization.
Ready to learn how we can streamline your cell therapy development?
Accelerate Your Pathway to Development - Contact Our Business Development Team Today





