At MaxCyte, we provide unparalleled regulatory support to help you navigate the complexities of global regulatory requirements with confidence.
Backed by over 20 years of experience and an FDA Master File continuously updated since 2002, our expert team supports successful submissions worldwide. Whether you're advancing an IND, CTA or BLA application, we offer comprehensive geographic coverage, flexible filing options, and tailored documentation to streamline your path from concept to commercialization. Trust MaxCyte's proven expertise to accelerate your programs and achieve regulatory success.
Advance your programs confidently with expert regulatory support from concept to commercialization and beyond.
Advance Efficiently with Flexible Regulatory Support
MaxCyte offers flexible regulatory support designed to meet the unique needs of your submission process. Whether you're filing an IND, CTA, or marketing application, our expertise ensures a seamless experience. With the flexibility to reference existing master files, create specialized technical documentation, or engage with regulatory agencies in new regions, MaxCyte is your partner in minimizing risk and accelerating approvals.

Regulatory support for the only electroporation platform with a commercial therapy approved.
Used in the manufacturing of Vertex Pharmaceuticals and CRISPR Therapeutics' groundbreaking CRISPR/Cas9 gene-edited cell therapy, CASGEVY®.
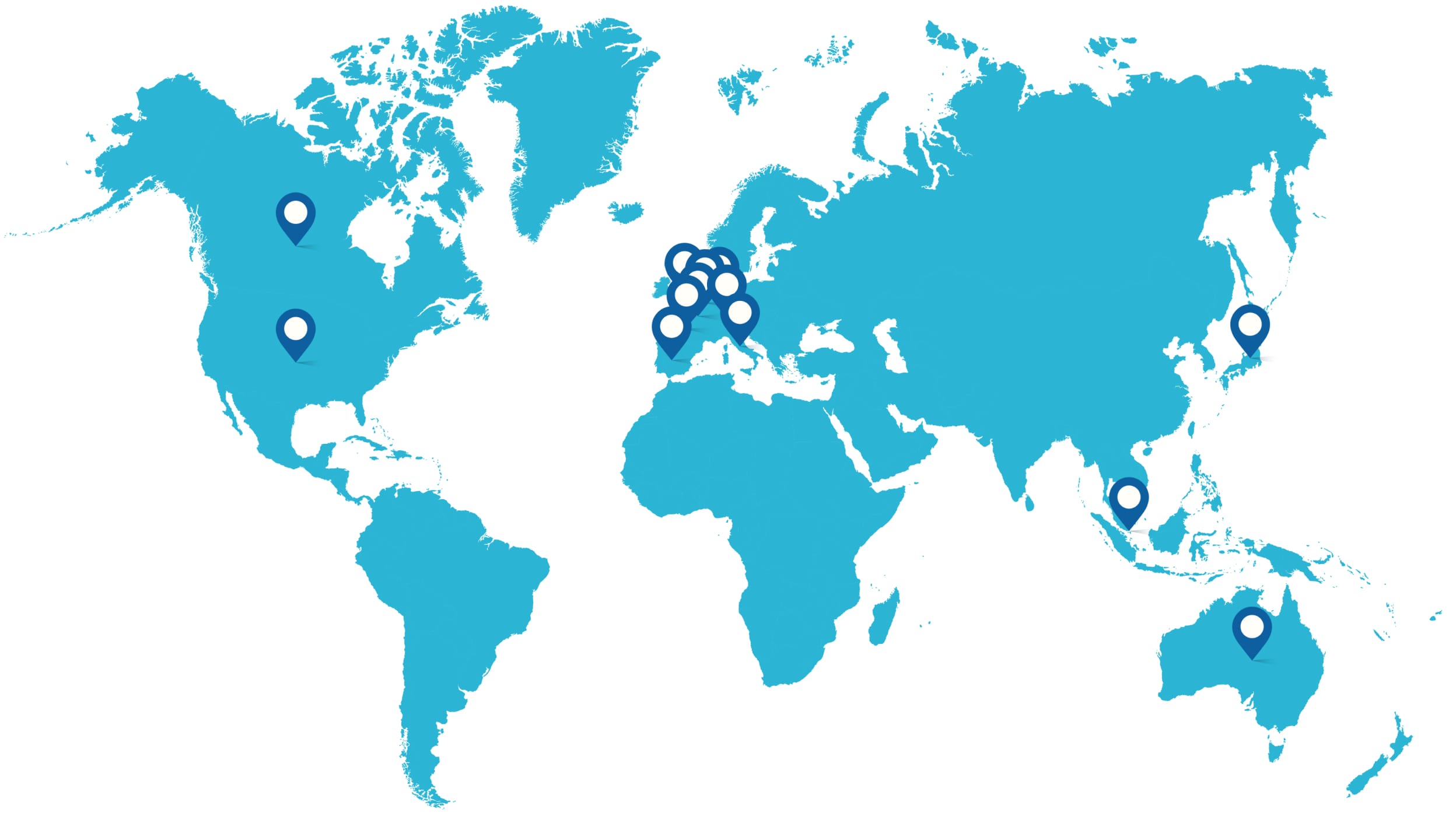
We've completed successful filings with agencies in regions that include:
- Canada
- Australia
- Switzerland
- UK
- Austria
- Belgium
- France
- Germany
- Italy
- Netherlands
- Spain
- Japan
- Singapore
We have a US FDA master file and global technical files that streamline the regulatory submission process and include all proprietary components intended for use in GMP-compliant manufacturing-instrument, buffer and processing assemblies.
70+
Clinical Trials
From INDs referencing
MaxCyte's master file
We have the flexibility to support your regulatory submission with the information that best meets the needs of your specific filing, whether it's an IND, CTA or marketing application.
We can:
- Reference existing master or technical files
- Provide customized documentation to submit special purpose technical files or to interact with a regulatory agency as needed
- Engage with regulators for filings in new regions.
Comprehensive Quality Support for Audit and Compliance
MaxCyte provides unparalleled support for manufacturing site audits and ensures compliance across regulatory landscapes.
Audit and Compliance Highlights:
- Over 20 successful audits with zero major non-conformances.
- On-demand expert support during manufacturing audits, with response times within 1 hour.
- Tailored audit readiness support, including IQ/OQ validation and annual PM for electroporation equipment.
- Seamless preparation and submission of regulatory documentation tailored to IND, CTA, or BLA filings.
- Proactive contingency planning to minimize potential downtime in manufacturing operations.
- A proven track record of assisting clients through challenging audit scenarios.

Customer-Centric Compliance Services:
- Detailed support for process validation and comparability assessments.
- Comprehensive training programs for manufacturing staff to ensure GMP compliance.
- On-site observation during engineering and manufacturing runs to validate audit readiness.


Advance Efficiently with Trusted Quality Systems
At MaxCyte we take pride in ensuring quality and compliance every step of the way, from concept to commercialization.
Discover how MaxCyte's robust quality systems ensure consistency, compliance, and excellence at every stage of your therapy's journey. From audit-ready facilities to proven GMP standards, our commitment to quality is unparalleled.






