Brochure
Accelerate Antibody Development and Production
Why does transfection matter?
Different modes of protein expression can be used throughout the discovery and development of recombinant therapeutic antibodies. With the right transfection method, you can produce consistently high-quality antibodies and proteins using the approach that fits your stage in development and helps you to achieve your goals faster. The MaxCyte ExPERT electroporation platform, supported by a global team of R&D and application scientists, can help accelerate your therapeutic antibody discovery and development to bring new treatments to patients sooner.
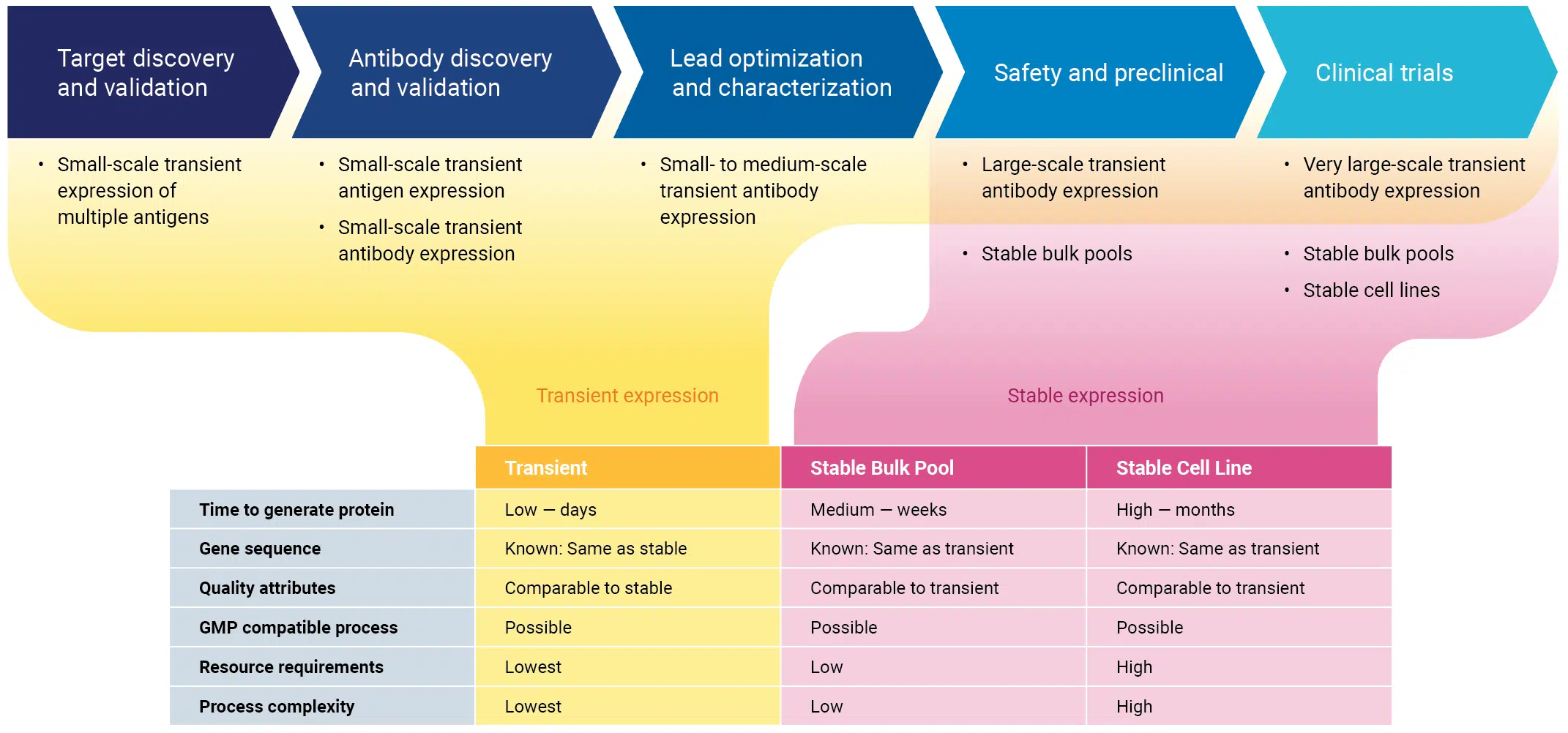
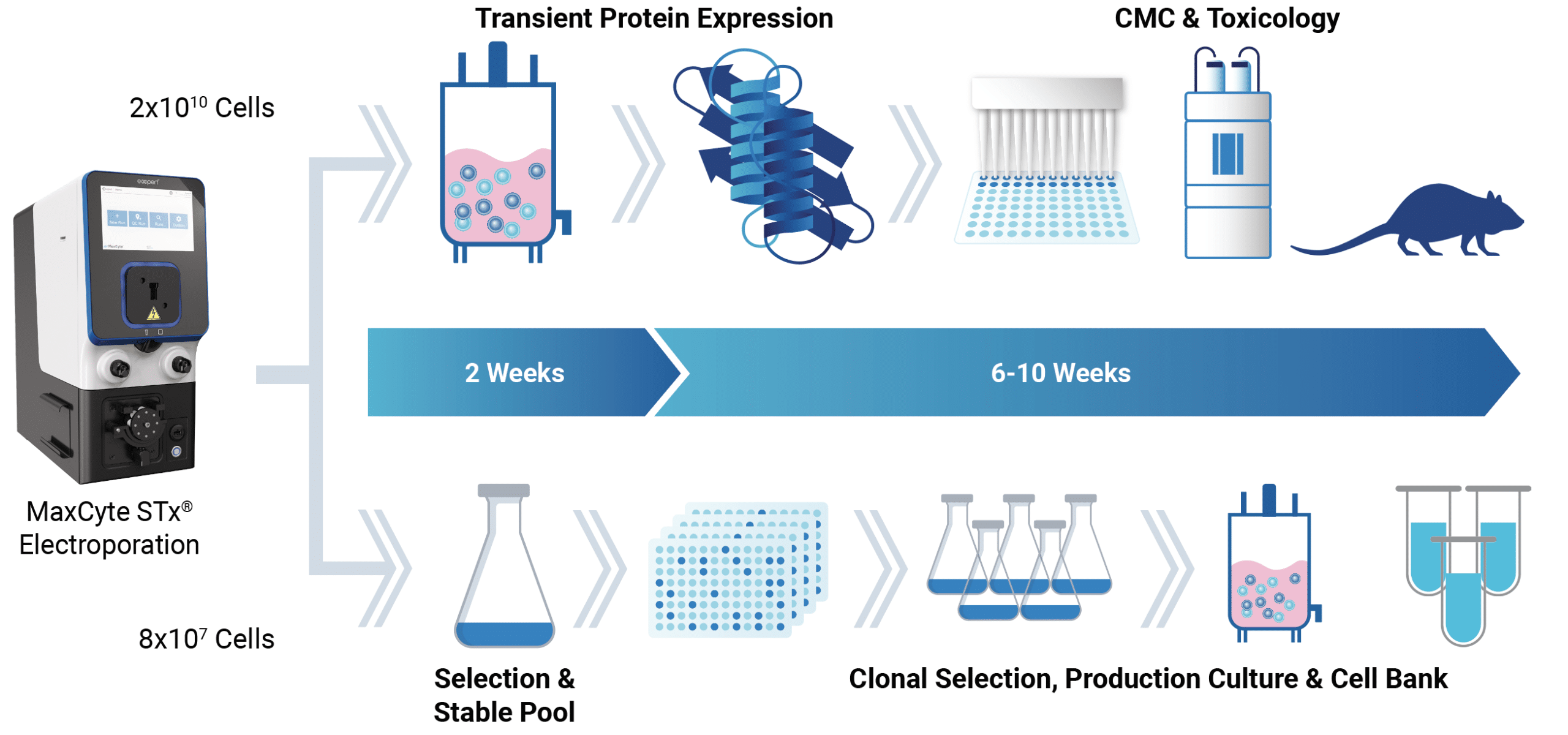
Protein expression for every stage of therapeutic antibody development
Electroporation can be used to transfect cells. An electrical pulse, or pulses, causes temporary and reversible membrane relaxation, allowing entry of molecules such as nucleic acids into the cell. With MaxCyte, you can transfect from 75,000 up to 700 million cells with static electroporation, or over 100 billion cells with our Flow Electroporation technology.
Transfection is the first step in producing recombinant protein by transient expression, stable bulk pools or stable cell lines, so your choice of transfection technology is critical for success.
The MaxCyte platform comprises electroporation instruments, optimized electroporation protocols, processing assemblies (electroporation consumables) and a single, universal electroporation buffer developed to deliver best-in-class transfection performance.
MaxCyte electroporation combines unmatched scalability and transfection efficiency in virtually any cell line so you can use your manufacturing host cells for antibody expression from discovery to stable cell line development.
Continuity from R&D to commercial manufacturing enables you to:
- increase productivity to shorten development timelines
- eliminate problematic leads sooner so you can focus on the best candidates
- accelerate development by avoiding repeated re-optimization or revalidation
- reduce the risk of candidate failure
Whatever cell types you use and whatever antibody you express, your choice of transfection technology matters.
Be flexible: transfect any cell
When your transfection method combines high efficiency and cell viability, you can get high yields from transient expression in whatever cell line you prefer. With MaxCyte electroporation, you can:
- transfect any cell type including adherent, suspension and custom cell lines
- use a single, chemically-defined electroporation buffer
- select the pre-loaded, electroporation protocol that has been optimized for your cell type
When you have the flexibility to generate high yields of protein by transient expression in the same cell type you use for stable cell line development, you can save time and resources by avoiding the need to reoptimize and revalidate.
Using the same cell type from discovery to manufacturing can streamline the identification, development and commercialization of antibodies of the highest quality.
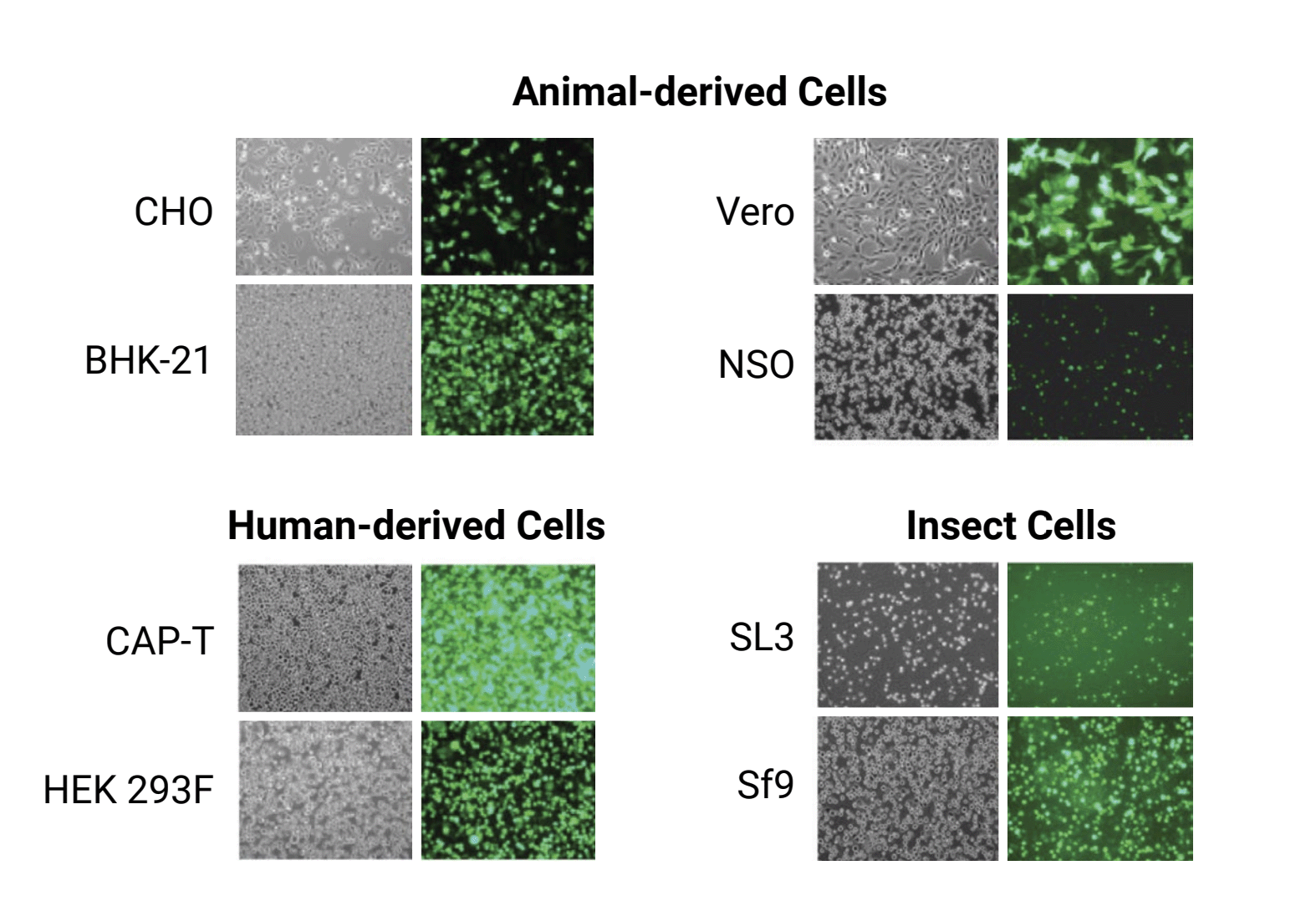
A simple workflow to transfect any cell type for antibody production

Be flexible: transfect cells at any scale
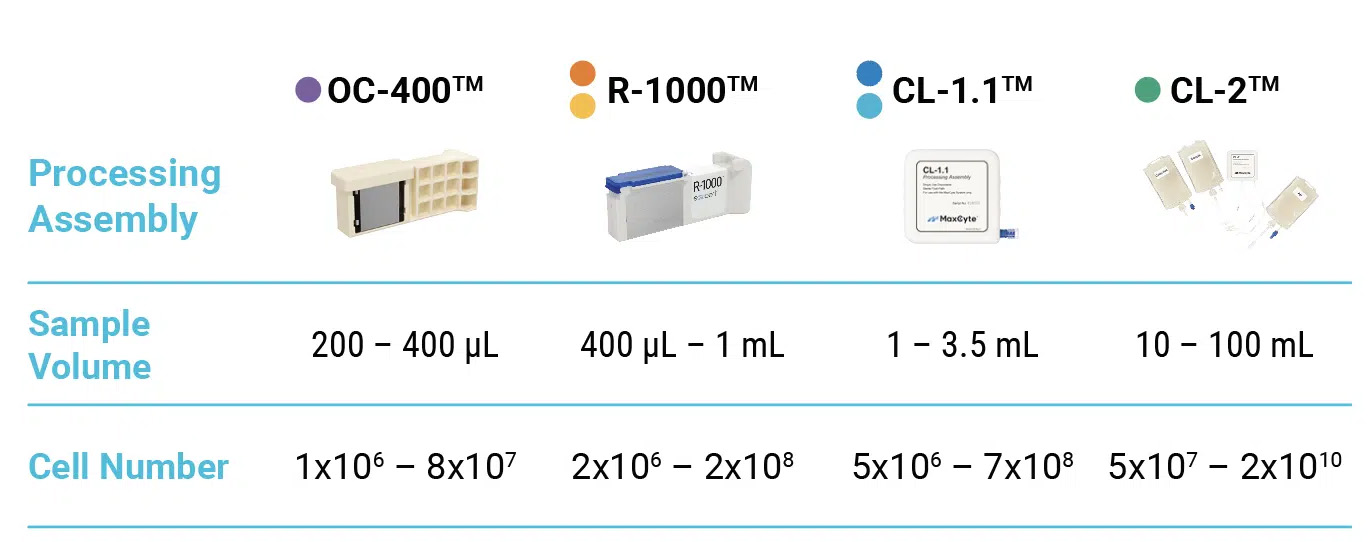
From 75,000 to over 100 billion cells, consistent electroporation conditions yield reproducible results. With our range of processing assemblies available for research use only or for GMP applications, we’ve got you covered at every scale for every stage of development.
Having the flexibility to transfect cells at any scale enables you to save time and resources by avoiding the need to re-optimize when you:
- switch between MaxCyte instruments
- optimize your process at small scale then scale up to produce more protein
- transfer processes between groups or sites
Electroporate from 75,000 cells to 700 million cells with traditional static electroporation, or 200 million cells to 200 billion cells with our Flow Electroporation technology. MaxCyte electroporation delivers consistent transfection conditions for consistent performance with all of our instruments and with static electroporation and our Flow Electroporation technology.
Save time and resources by avoiding costly re-optimization and advance your project without delays.
See the full range of electroporation consumables, known as processing assemblies (PAs).
Be flexible: express any type of protein
The development of therapeutic antibodies isn’t just about producing IgG. You may need to express appropriate antigens as well as lead candidates, and while most approved antibody therapeutics are standard IgGs, there is increasing interest in novel antibody formats, fragments and fusions. Expressing these non-natural proteins may increase cell stress, potentially reducing productivity. MaxCyte electroporation preserves cell viability and can deliver high transient yields of standard and novel antibody formats, antigens and other recombinant proteins.
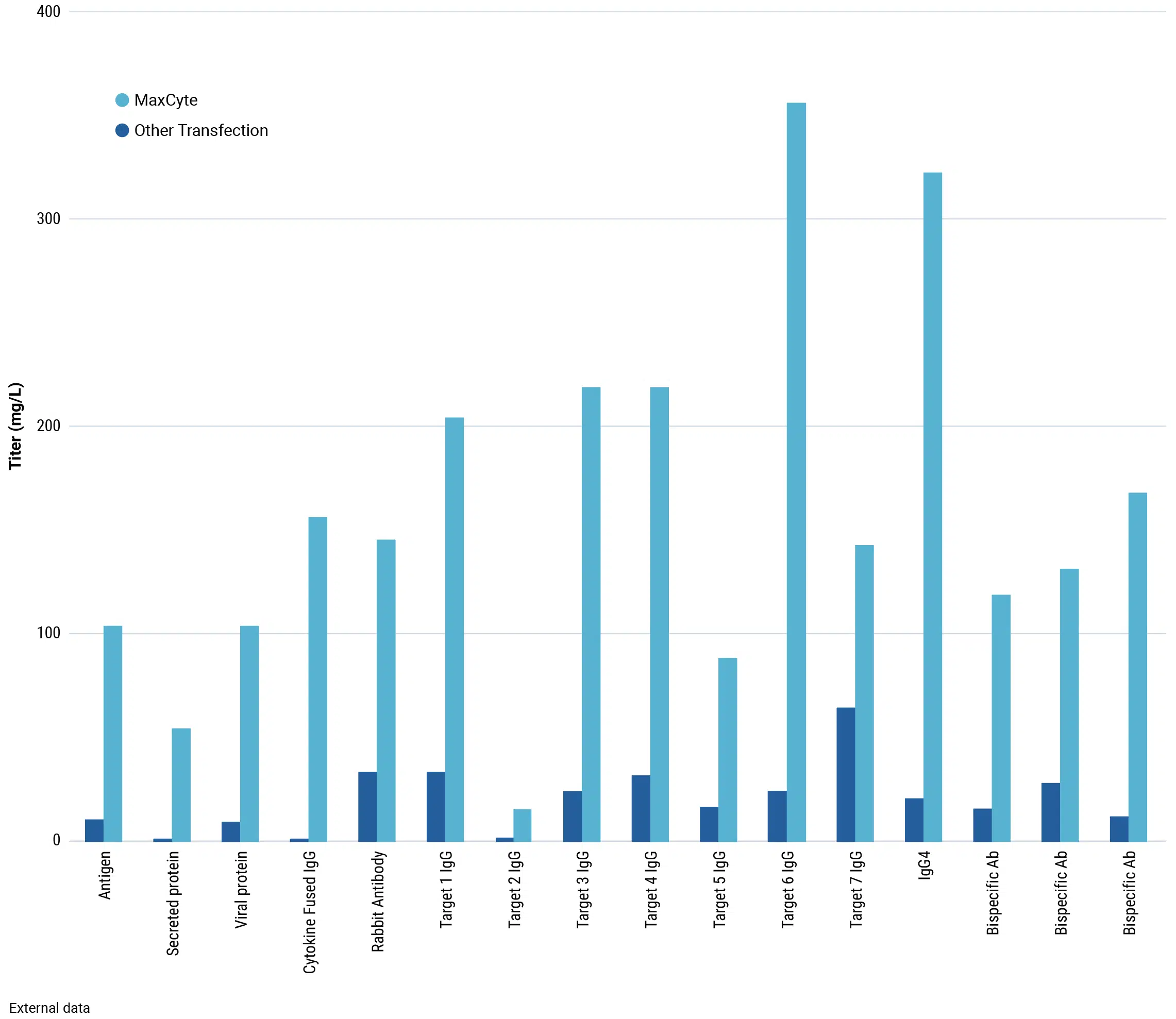
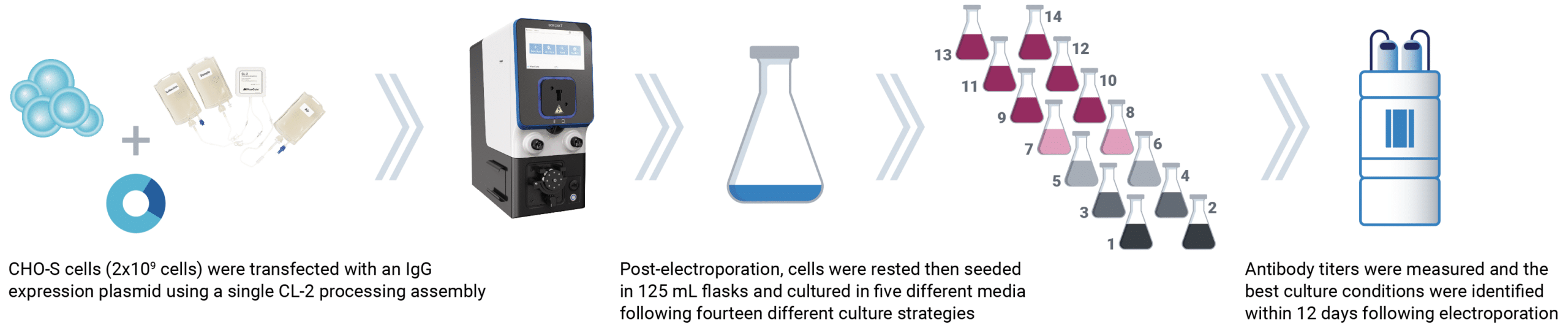
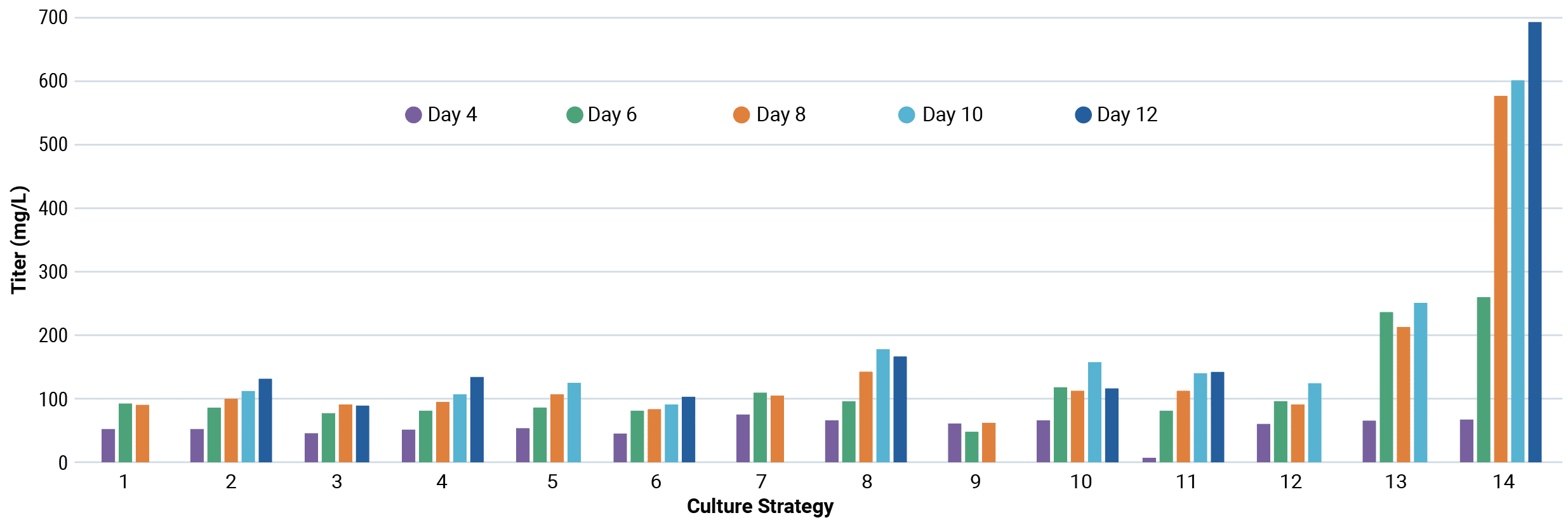
Be flexible: use the culture strategy you want
When you combine high-efficiency transfection with high cell viability, you can be more flexible than ever before. Achieve high antibody yields without the need for specialized proprietary enhancers, plasmid vectors, or culture media.
Cells transfected with MaxCyte electroporation can produce high levels of protein with alternative media and feed formulations, enabling you to use the combination that achieves the cost savings, process intensification or simplified purification strategy you need.
Workflow for media strategy optimization
Be fast: rapid transient antibody production
Produce grams of high-quality protein by transient expression within days after MaxCyte electroporation, because speed is important, and you don’t have to compromise product quality to get it. With MaxCyte electroporation for transient antibody expression you can:
- express grams of antibody in less than fourteen days after electroporation instead of waiting weeks or months for material from stable cells
- save time with reproducible performance from 75,000 cells to 2×1010 cells, for scale-up without re-optimization
- produce protein with consistent quality between transient and stable expression for consistent product performance, from discovery to final manufacturing
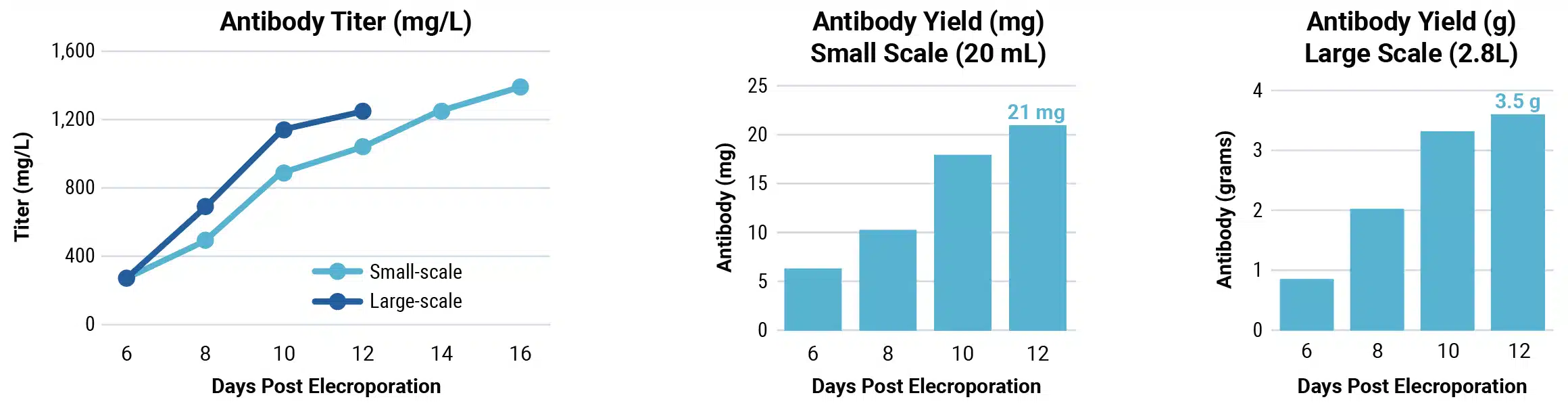
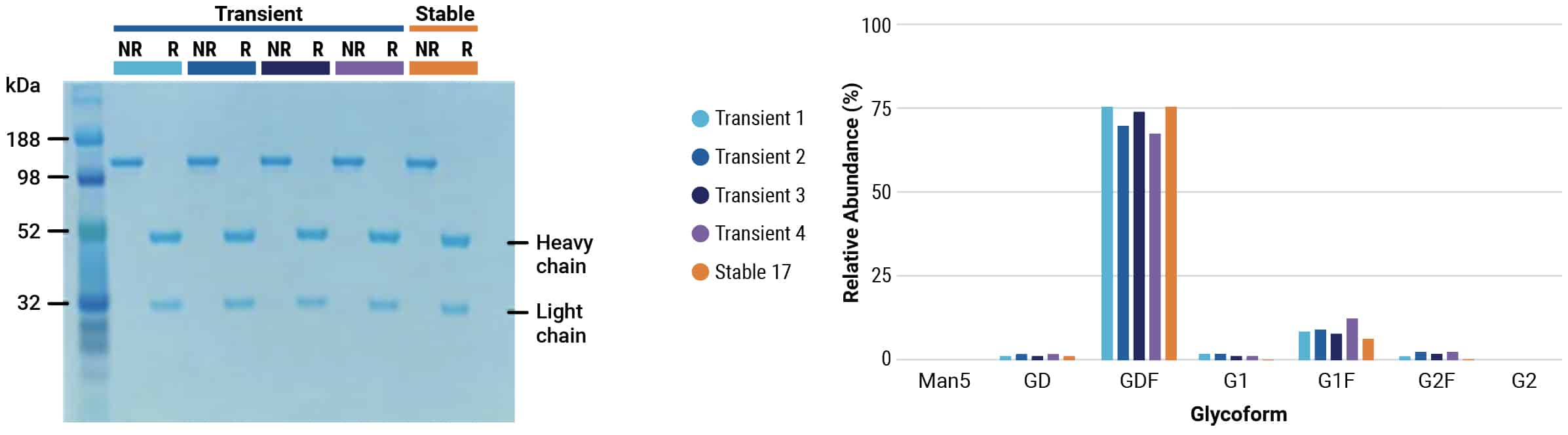
Be fast: large-scale transient antibody expression with the ExPERT VLx
Scalable electroporation can produce IND-enabling IgG yields in under four weeks
- Produce multi-grams of protein in less than a month from initial vial thaw to protein harvest
- Benefit from reproducible performance at any scale from 75,000 cells to 1×1011 cells
- Generate high-quality protein produced by large-scale transient expression
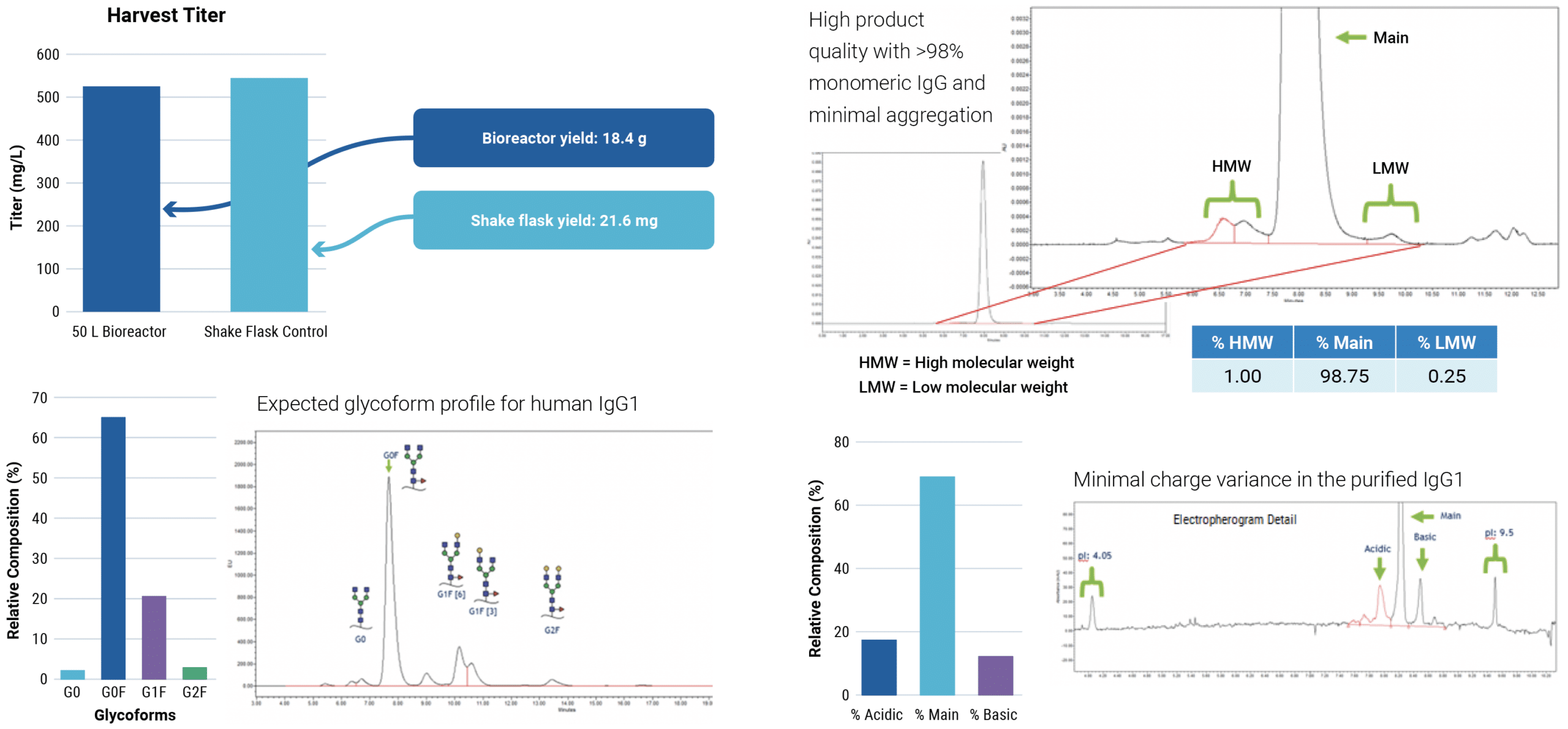
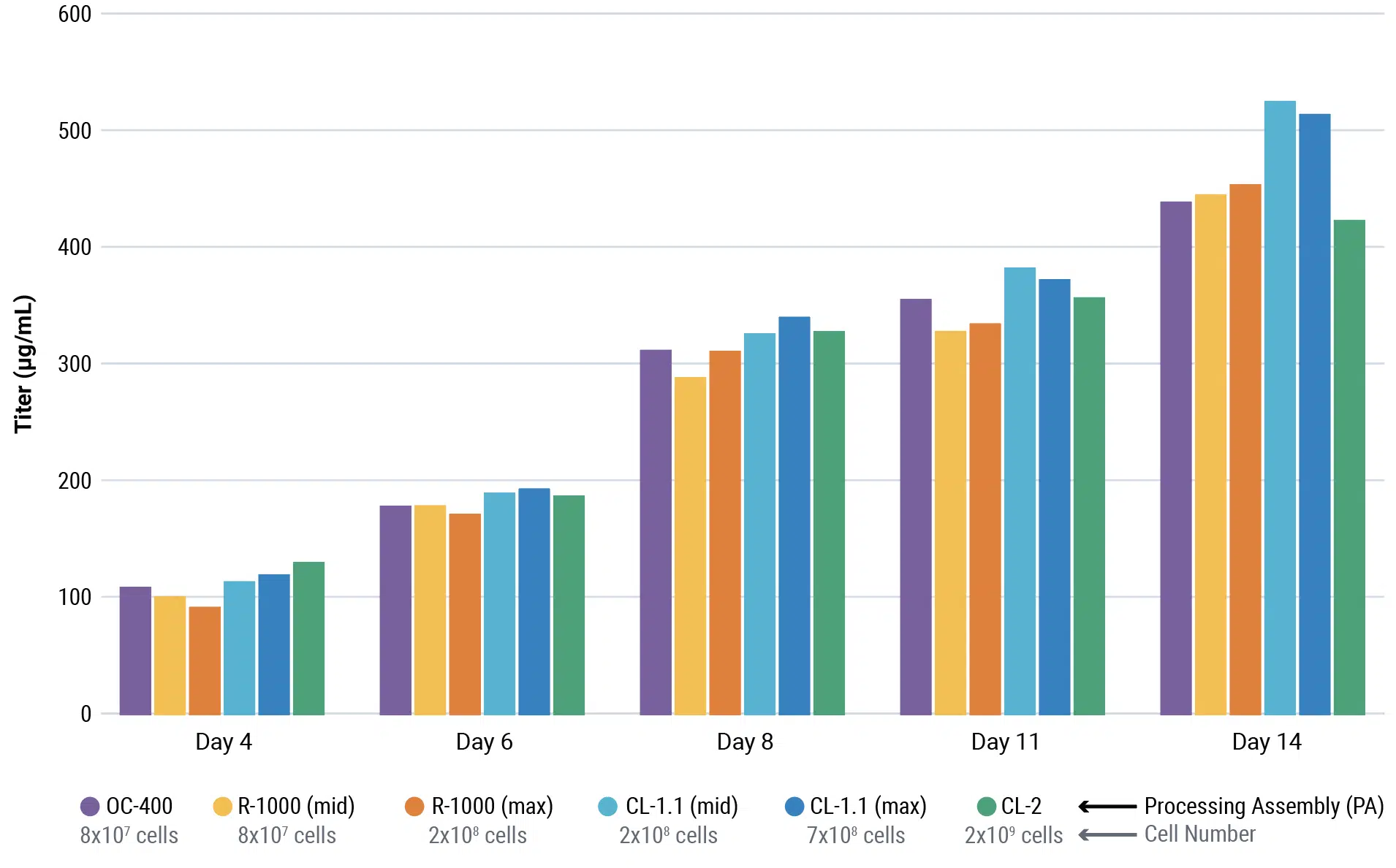
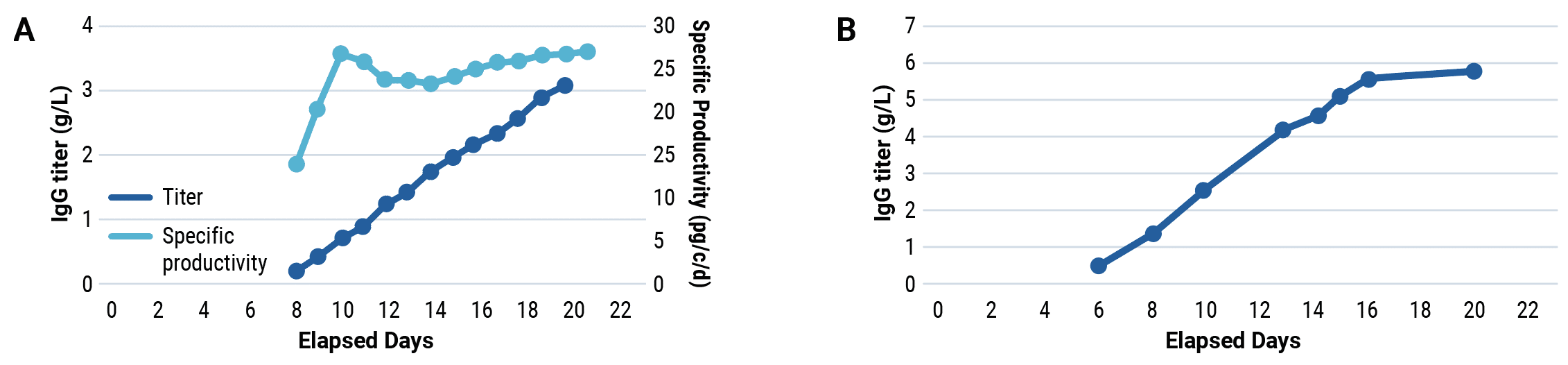
CHOZN™ cells were electroporated with a human IgG1 expression plasmid in a 1 mL or 1 L processing assembly on the ExPERT VLx. Production cultures were maintained in a shake flask or bioreactor. Protein was harvested when culture viability dropped to 50%. Total yield was quantified and protein quality was assessed.
Be fast: stable bulk pool development
Stable bulk pools are a faster alternative to stable cell line development to produce antibody material for IND-enabling and early clinical studies, but the selection process can still take several weeks.
MaxCyte electroporation delivers high transfection efficiency without compromising cell health, so you can start selection sooner or with higher stringency than with chemical transfection. MaxCyte electroporated cells recover from selection faster than with other transfection methods, enabling rapid, high-yielding stable bulk pool development.
Bulk pools by random integration
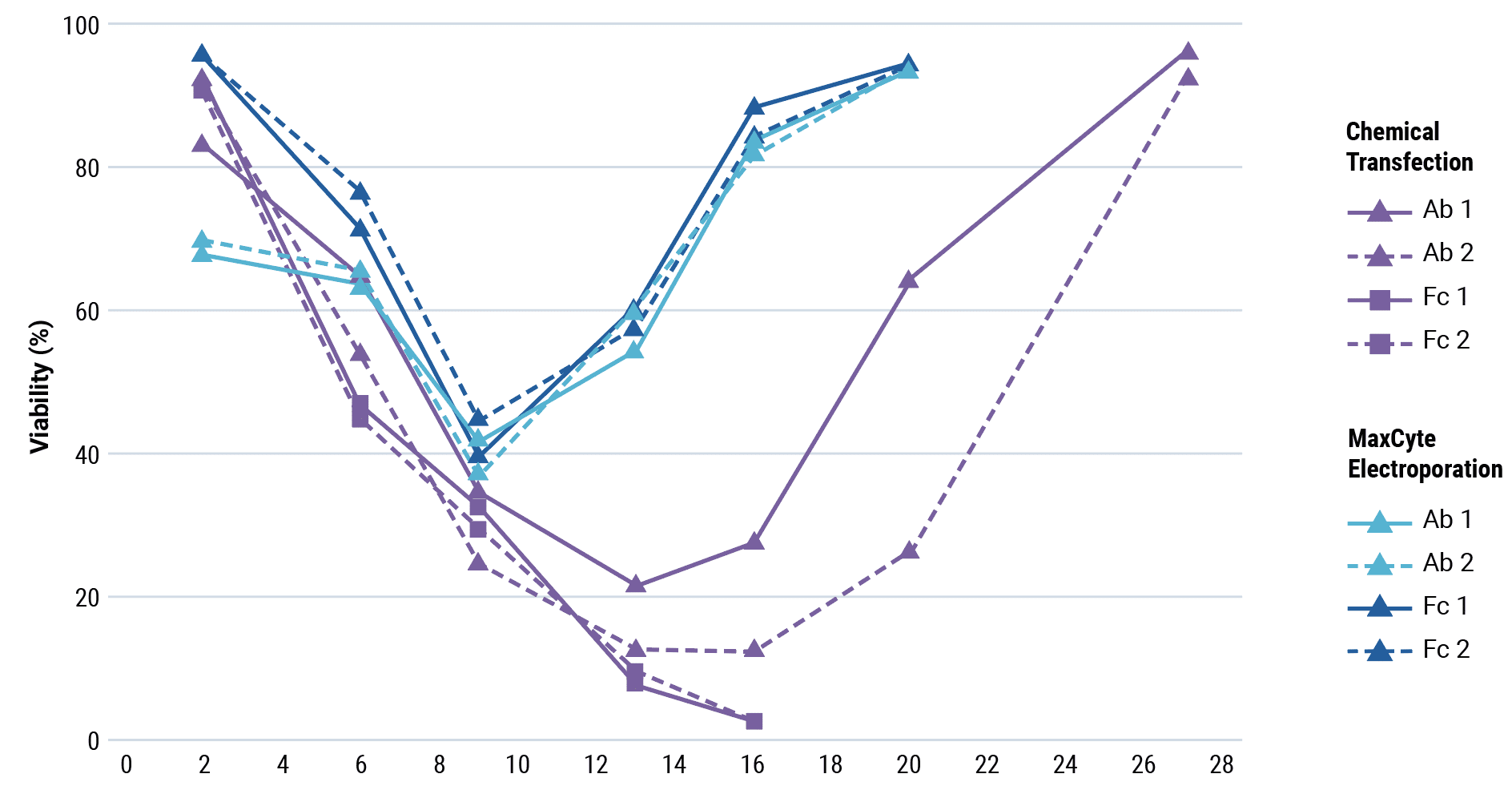
Bulk pools by transposase-mediated transposon integration
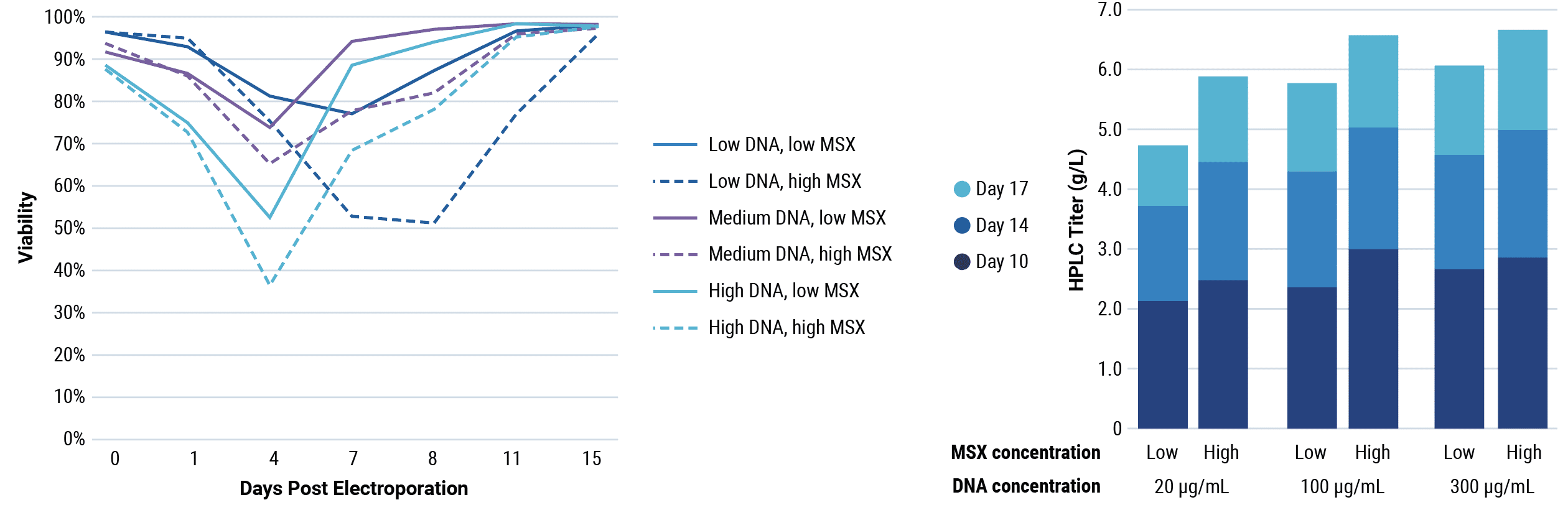
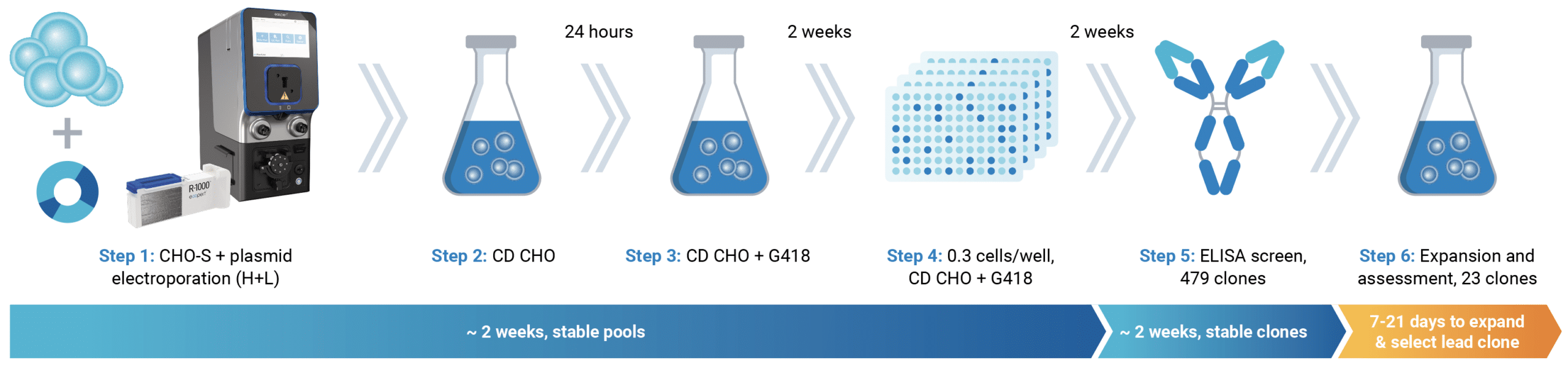
Be fast: stable cell line development
When it’s time to develop a stable monoclonal cell line, the high-transfection efficiency and cell viability delivered by MaxCyte electroporation can accelerate your workflow by enabling you to:
- start selection sooner because electroporated cells have high viability
- subclone earlier because pools recover faster from selection
- use more stringent selection and screen fewer colonies to identify high-yielding clones
Be first: transient antibody expression for IND-enabling studies
The traditional approach to therapeutic antibody development
Transient gene expression is routinely used in discovery and early development when recombinant antigens and low milligram quantities of several antibody candidates are required. Cells are transfected with expression vectors, usually plasmid DNA, and protein is generated by small-batch culture of the transfected cell pool without selection. The cells used at this stage may not be the same cells used for final product manufacturing, necessitating revalidation later on.
Following the traditional approach, it can take 6 to 12 months to develop a stable cell line and express sufficient material for IND-enabling studies.
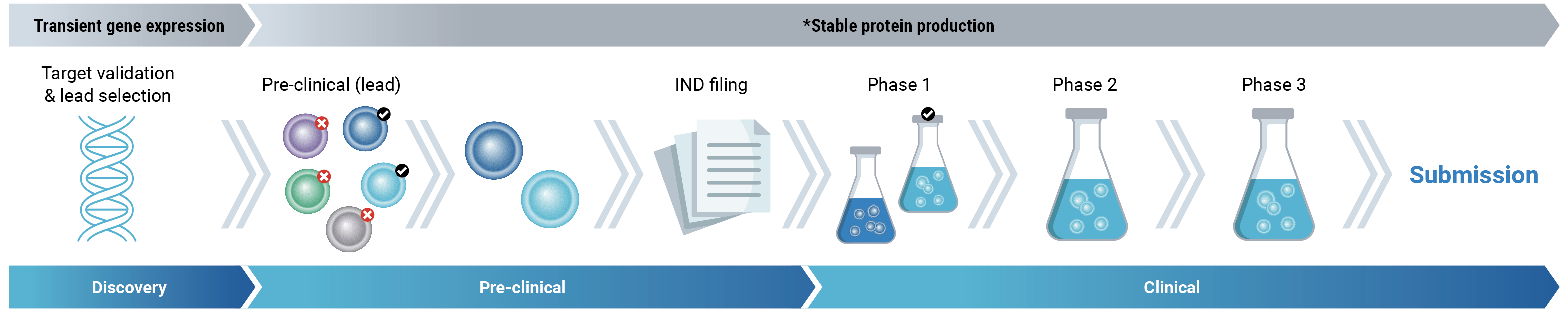
As development advances and candidate selections proceed, researchers often transition to stable expression in their preferred manufacturing cell line due to the increasing need for more material. Stable pools, a straightforward method for achieving stable protein expression, usually yield higher quantities than transient gene expression and require less time than the development of stable cell lines. Transfected cells undergo selective pressure and are subsequently cultured to facilitate protein production.
Ultimately, the development of stable monoclonal cell lines becomes imperative to ensure an adequate protein supply for the final stages of development and subsequent commercial manufacturing. Despite the advent of numerous new technologies, the process of stable cell line development remains a time-intensive and expensive endeavor, posing potential barriers to progress.
A better way: transient gene expression for accelerated therapeutic antibody development
Therapeutic antibody developers could reduce the time to IND-filing while cutting costs by using transiently expressed protein for more steps in the development workflow. To enable the use of this transformative strategy, a transient gene expression approach capable of generating multiple grams of protein is essential. MaxCyte electroporation can efficiently transfect over 100 billion cells in a single reaction, generating tens of grams of protein within two weeks, enabling efficacy, toxicology, and developability studies to be carried out without the need for stable cell lines.
Transient gene expression can produce IND-enabling protein quantities within 4-6 weeks
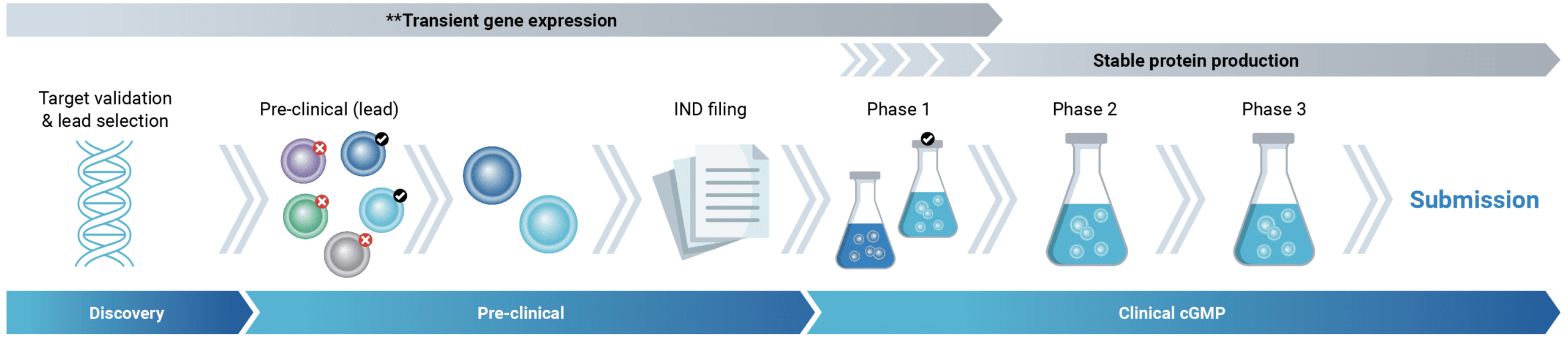
The ability to use transiently-expressed material for all pre-IND activities could enable more educated ‘go’ or ‘no-go’ decisions to be made faster, saving time and conserving resources. Transfection is a vital step in recombinant antibody production; and whatever mode of expression you use, the transfection technology you choose matters.
Be first: with parallel development
Sometimes, speed is everything
When getting to the market first is the top priority, MaxCyte enables a paradigm-shifting, parallel development approach to speed your journey to the clinic. Empowered with the ability to efficiently transfect up to 20 billion cells in one reaction with the MaxCyte STx, or over 100 billion cells with the ExPERT VLx, developers can choose to prioritize speed by splitting electroporated cells between production culture and stable cell line development. Transiently expressed protein can be used for analytical and process development, CMC and toxicology studies in parallel with stable cell line development.
MaxCyte enables cell line development while you progress instead of ‘while you wait’ for the fastest route to IND
The ExPERTs in electroporation
Developed to deliver consistent performance from the first time you use it, the MaxCyte electroporation platform brings together our ExPERT instruments, consumables that work at any scale from 75,000 to 20 billion cells, and a gentle electroporation buffer suitable for any cell type.
Features
- Integrated touch screen: operates at the touch of a finger
- Enhanced software user interface: save time with updated software providing additional functionality and ease of use
- LED status indicators: quickly visualize instrument and run status with six colorful and clearly-defined status modes
- Reduced footprint: bench-scale, modular equipment
- Elegant design: enjoy a modern, sleek appearance that fits seamlessly into any high-tech lab space
- Network capable: generate and save run reports automatically onto a shared local drive
Electroporation consumables
ExPERT processing assemblies and electroporation buffers provide researchers, production scientists, and GMP facilities with an optimized vehicle to transfect cells, achieving required levels of efficiency, viability, and consistency to reach project goals and milestones as quickly as possible.
The ExPERT VLx: for multi gram-scale protein production
The ExPERT VLx system offers unparalleled transfection scalability through Flow Electroporation technology, ensuring industry-leading reproducibility, flexibility, and ease of use, thus accelerating biotherapeutic development by enabling the production of multi-gram protein quantities through transient expression.
Features
- Scalable: Transfect over 100 billion cells in a fully closed, single-use system in less than 30 minutes
- High performance: Achieve reproducible results with superior transfection efficiency, cell viability, and protein expression, even in difficult-to-transfect cell lines
- Versatile: Bench-scale, modular equipment with automated flow design, intuitive integrated software and user-friendly open architecture
- Closed-system connectivity: Sterile bioweldable connections on the R1-L processing assembly and the VLx collection manifold tubing set, enabling three VLx instruments to operate in parallel
Benefits
- Reduce time, cost, and risks
- Obtain grams of proteins in weeks, not months
- Scale seamlessly from discovery to manufacturing
- Expedite your transition from development to Phase I
- Accelerate your path to the clinic
- Parallel transfection for virtually any transient production need
Request a quote
Speed up your protein production with the MaxCyte ExPERT electroporation platform. Contact our support team so they can build you a quote.
References
- Reproduced from J Biomol Screen, 20(4), Krista Steger, James Brady, Weili Wang, Meg Duskin, Karen Donato, Madhusudan Peshwa. CHO-S Antibody Titers >1 Gram/Liter Using Flow Electroporation-Mediated Transient Gene Expression followed by Rapid Migration to High-Yield Stable Cell Lines. 545-551., Copyright (2015) with permission from Elsevier.
- Reproduced from J Biomol Screen, 20(4), Krista Steger, James Brady, Weili Wang, Meg Duskin, Karen Donato, Madhusudan Peshwa. CHO-S Antibody Titers >1 Gram/Liter Using Flow Electroporation-Mediated Transient Gene Expression followed by Rapid Migration to High-Yield Stable Cell Lines. 545-551., Copyright (2015) with permission from Elsevier.