Application Note
Engineering NK Cells with High-Affinity CD16 for Enhanced Combination Therapy to Treat B-Cell Lymphoma
Background
NK cells play a pivotal role in rapid and highly efficient cancer surveillance and represent a promising avenue for adoptive cell transfer either as a mono- or combination immunotherapy. Early studies on the transfer of unmodified autologous or allogeneic NK cells have established their clinical safety and demonstrated modest anti-tumor efficacy.1,2
One mechanism of NK cell anti-tumor activity is antibody-dependent cellular cytotoxicity (ADCC) induced via Fc receptor (CD16) binding to antibodies. Patients homozygous for a natural CD16 polymorphism, CD16-158V which displays higher IgG1 and IgG3 affinity, have improved clinical outcomes upon anti-tumor therapeutic antibody treatment.3-7
Only 10% of the human population is homozygous for this polymorphism, suggesting that genetic manipulation of NK cells to express CD16-158V prior to adoptive transfer may improve clinical success not only of NK cell therapies but also anti-tumor IgG1 antibodies. 8
In vitro studies have demonstrated that NK cell lines engineered to overexpress CD16-158V have improved cytotoxicity against antibody-coated cancer cell lines supporting genetic reprogramming of NK cells; however, primary NK cells have historically been difficult to engineer.9,10
Non-viral engineering of NK cells using MaxCyte® mRNA electroporation provides significant benefits including high efficiency and low toxicity as well as clinical scalability enabling rapid development of novel adoptive cell therapy approaches.11-13
Aim
Establish overexpression of high-affinity CD16 on NK cells as a potential combination therapy for improving ADCC activity of anti-tumor monoclonal antibodies. Specifically, demonstrate efficient expression of high-affinity CD16 in NK cells with minimal effects on cell viability and phenotype using mRNA electroporation and assess the effects of CD16-158V expression on rituximab - mediated cytotoxic activity against B cell lymphoma cells.
NK Cell Electroporation
-
- NK cells were isolated from healthy peripheral blood mononuclear cells (CD16-158F/F donors) and expanded ex vivo for 11-15 days with irradiated EBV-SMI-LCL cells.
- Cells were resuspended in 100 µL of MaxCyte® Electroporation Buffer containing various concentrations of mRNA encoding the high-affinity Fc receptor (CD16-158V) and electroporated on the MaxCyte GT®.
- Electroporation was performed using MaxCyte recommended protocol.
- Post electroporation, cells were resuspended in NK cell media and transferred to culture flasks for in vitro analysis.
Full methods for ex vivo NK cell expansion and in vitro assays are detailed in Front. Immunol., 7, 105, 2016.
Results
Highly Efficient CD16-158V Engineering of Human NK Cells
Ex vivo expanded NK cells electroporated with mRNA encoding the high-affinity Fc receptor, CD16-158V, demonstrated a significant increase in CD16 surface expression (Figure 1A). CD16 expression correlated with the concentration of CD16-158V mRNA used during electroporation and ranged from a 2-fold increase using 1 µg/106 NK cells up to a 3-fold increase using 8 µg/106 NK cells (data not shown).
CD16 expression peaked 24 hours post electroporation and remained higher than non-electroporated NK cells for up to 72 hours post electroporation (Figure 1B). While increased CD16 expression was transient in nature, this may not negatively impact the therapeutic potential of engineered NK cells due to the short persistence of adoptively transferred NK cells. Importantly, no major changes in NK cell phenotype, as measured by expression of 15 inhibitory or activating surface receptors, were observed following electroporation with CD16-158V mRNA.13
Significantly Increased CD16 Expression
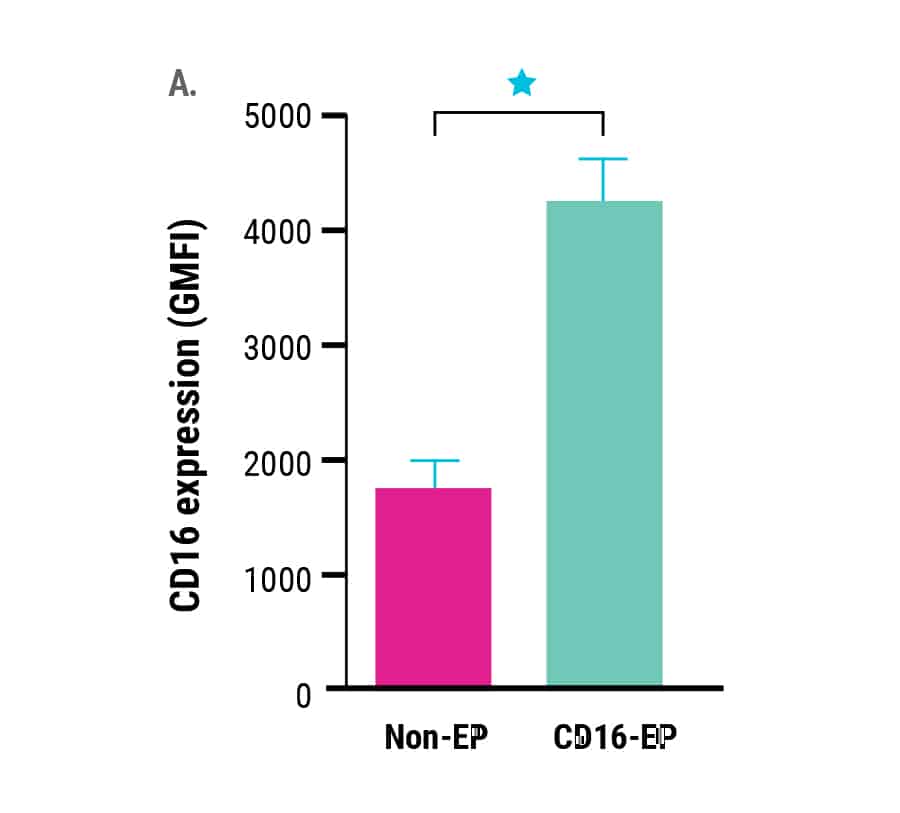
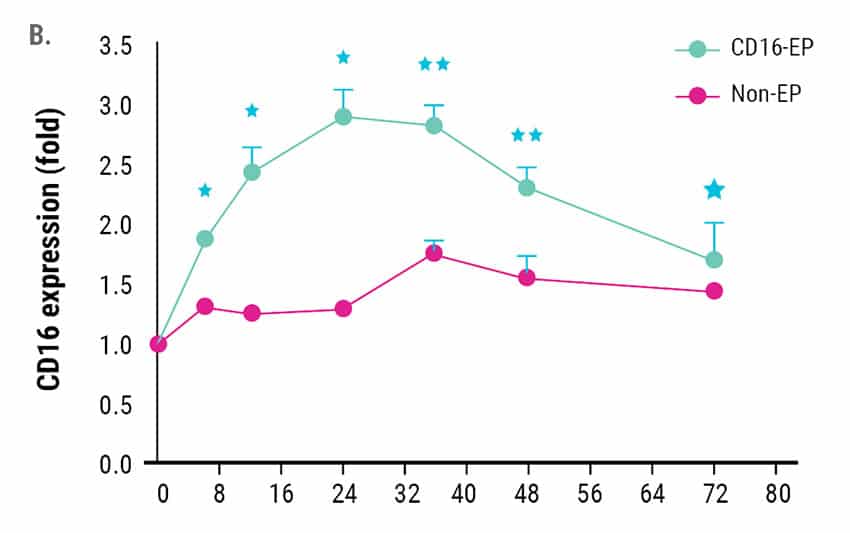
Figure 1. Ex vivo expanded NK cells were electroporated with mRNA (4 µg/106 NK cells) encoding the high-affinity, CD16-158V Fc receptor. NK cell CD16 expression was measured via flow cytometry for 72 hours post electroporation. Non-electroporated, ex vivo expanded NK cells were used as a control to assess endogenous CD16 expression. A) CD16 expression levels 24 hours post electroporation (n=7). B) Kinetics of CD16 expression (n=3). *p<0.05, **p<0.01.
Enhanced Rituximab-Mediated Anti-Tumor Cytotoxicity Upon NK Cell CD16-158V Expression
In vitro cytotoxic activity against rituximab-coated CD20+ B cell lymphoma cells of CD16-engineered or non-engineered NK cells was assessed 24 hours post electroporation. Engineered NK cells had an enhanced ability to mediate ADCC as measured both via NK cell degranulation (CD107a expression, Figure 2A) and specific lysis of B cell lymphoma cells (51Cr release assay, Figure 2B).
Augmented ADCC persisted for 3 days post electroporation, correlating with the kinetics of increased CD16 expression as well as the concentration of CD16-158V mRNA used for electroporation.13 Of note, mRNA electroporation did not negatively impact the NK cell non-ADCC-mediated cytotoxic function (data not shown).
Augmented Rituximab® Cytotoxicity
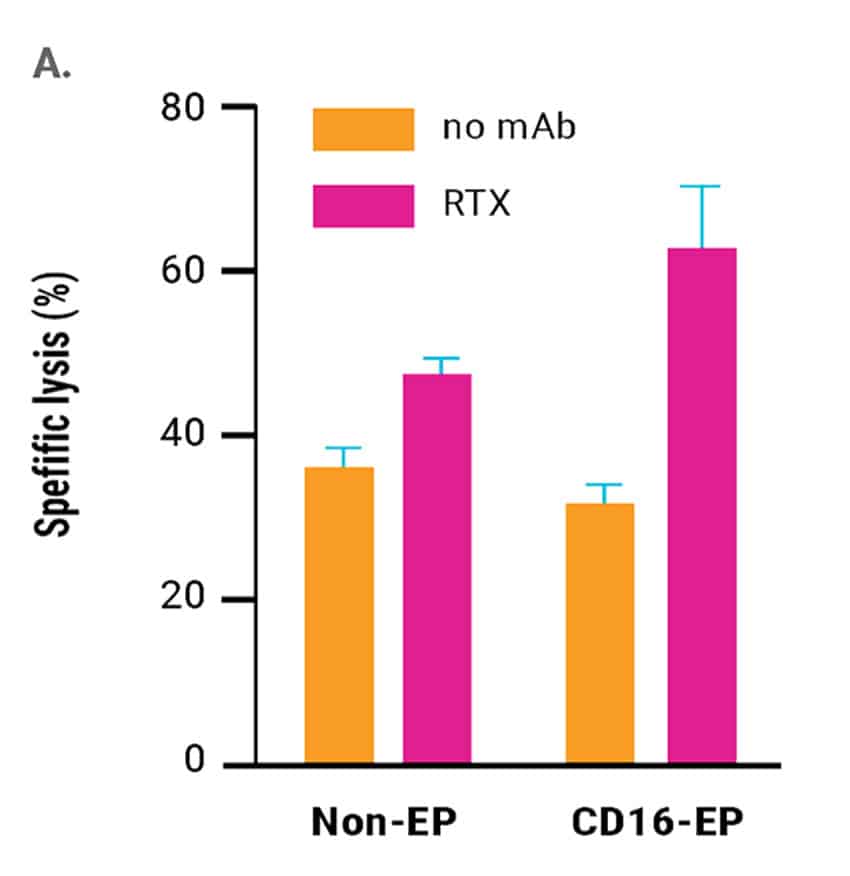
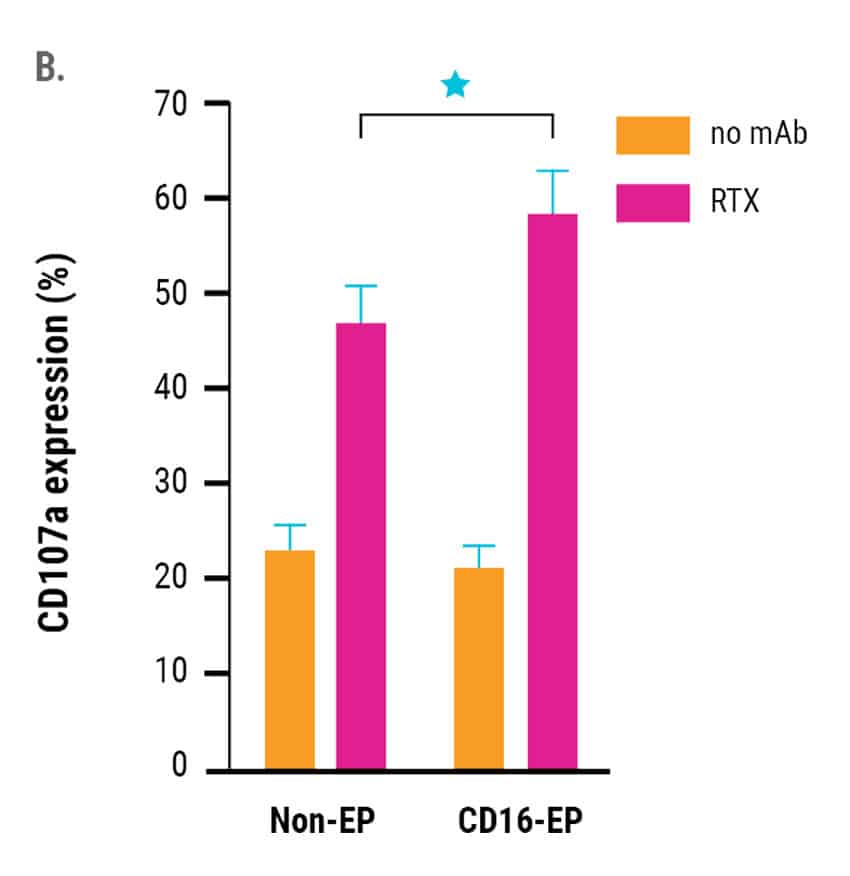
Figure 2. A) CD16-158V electroporated or non-electroporated expanded NK cells were cocultured with EBV-transformed B cell lymphoma cells (CD20+ 721.221 EBV-LCL cells) in the presence or absence of rituximab for 24 hours post electroporation. NK cell degranulation was assessed via CD107a expression within the CD56+ cell population. *p<0.05. B) Rituximab-mediated lysis of B cell lymphoma cells was measured in a 51Cr-release assay 24 hours post electroporation using a 0.5:1 ratio of electroporated or non-electroporated NK cells to EBV-LCL target cells.
Conclusion
MaxCyte’s solid foundation of efficiency, low toxicity, payload flexibility, and clinical scalability opens the doors to sophisticated, multi-tiered engineering of NK cell biology, to improve the efficacy and clinical success of adoptive NK cell therapies. The data summarized in this application note and in the associated published data, illustrate the use of mRNA electroporation via MaxCyte’s ExPERTTM instrument to rapidly and efficiently engineer ex vivo-expanded NK cells, while maintaining NK cell viability, phenotype and baseline cytolytic activity. The ability to genetically modify NK cells using a cGMP-compliant, clinical-scale method provides the opportunity to develop commercial cellular immunotherapies and combination immunotherapies against a range of cancers, including the expression the high-affinity CD16 Fc receptor, which in these preclinical studies proved to augment the anti-tumor activity of rituximab®, a currently approved biotherapeutic antibody.13
References
- Successful adoptive transfer of in vivo expansion of human haploidentical NK cells in patients with cancer. (2005) Blood, 105:3051-3057.
- Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. (2002) Science, 295:2097-2100.
- Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. (2002) Blood, 99:754- 758.
- Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. (2003) J. Clin. Oncol., 21:3940-3947.
- Polymorphisms in FcgammaRIIIa (CD16) receptor expression are associate with clinical response to rituximab in Waldenstrom’s macroglobulinemia. (2005) J. Clin. Oncol., 23:474-481.
- Correlation of FCGR3A and EGFR germline polymorphisms with the efficacy of cetuximab in KRAS wild-type metastatic colorectal cancer. (2010) Eur. J. Cancer, 46:1829-1834.
- Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. (2011) Blood, 118:3347-3349.
- A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. (1997) J. Clin. Invest., 100:1059-1070.
- Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. (2008) J. Immunol., 180:6392-6401.
- Genetic manipulation of NK cells for cancer immunotherapy: techniques and clinical implications. (2015) Front. Immunol., 6:266.
- Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. (2009) Cancer Gene Ther 17(3):147-54.
- A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. (2012) Cytotherapy, 14(7): 830-840.
- Efficient mRNA-based genetic engineering of human NK cells with high-affinity CD16 and CCR7 augments rituximab-induced ADCC against lymphoma and targets NK cell migration toward the lymph node-associated chemokine CCL19. (2016) Front. Immunol., 7:105.





