Application Note
High-Throughput Screening of Ion Channel Variants Using Automated Patch Clamp Recordings in Assay-Ready Cells
Aim
In the ground-breaking study summarized here, the authors presented a method for the high-throughput evaluation of the functional consequences of KCNQ2 ion channel variants.1 The technique was used to characterize 39 previously unstudied epilepsy-associated Q2 variants and their responses to treatment with a candidate therapeutic. MaxCyte® electroporation was used to produce Assay Ready Cells suitable for automated patch clamping, enabling ion channel variant characterization on an unprecedented scale.
Preparation of Assay Ready Cells
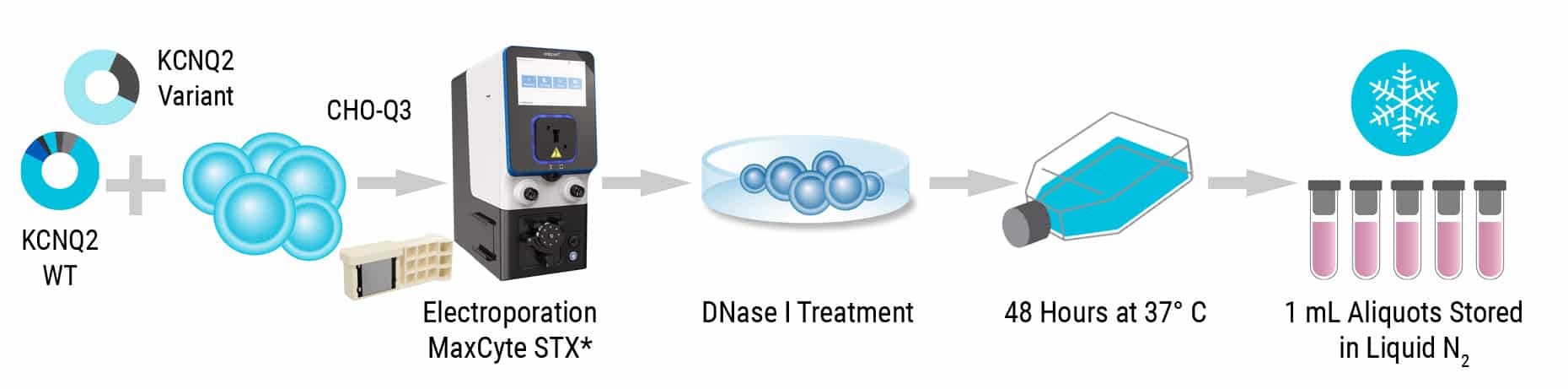
CHO cells stably expressing human KCNQ3 (CHO-Q3 cells) were electroporated with plasmids expressing Q2 variants to generate homozygous (variant Q2 alone) or heterozygous (variant Q2 + WT Q2; 1:1 ratio) KCNQ2 Assay Ready Cells.
Background
Ion channels are multi-subunit membrane proteins that regulate the passive flow of ions into and out of cells and their organelles; they are classified by the ion they are selective for and the mechanism that controls pore opening (gating). Pathogenic ion channel variants are found in individuals with various conditions, including epilepsy and cardiac disorders.
Kv7 is a family of tetrameric voltage-gated potassium channels encoded by five KCNQ genes, KCNQ1-5. The KCNQ2 subunit (Q2) can form a homomeric ion channel or co-assemble with KCNQ3 (Q3) to form heterotetramers widely expressed in the nervous system.2
Hundreds of Q2 genetic variants are associated with epilepsy and neurodevelopmental disorders; several of these have been characterized functionally but far more remain to be studied. As potentially life-changing precision medicines are developed, there is an even greater need to classify ion channel variants so that patients who might benefit from new drugs can be rapidly identified. Increased genetic screening of patients has led to the identification of more candidate pathogenic variants than can be studied using conventional techniques. The day-to-day variability inherent in traditional, labor-intensive manual methods is exacerbated when studies are completed in different labs. A simple, consistent expression system compatible with assay automation will be invaluable to generate reliable data on hundreds of variants.
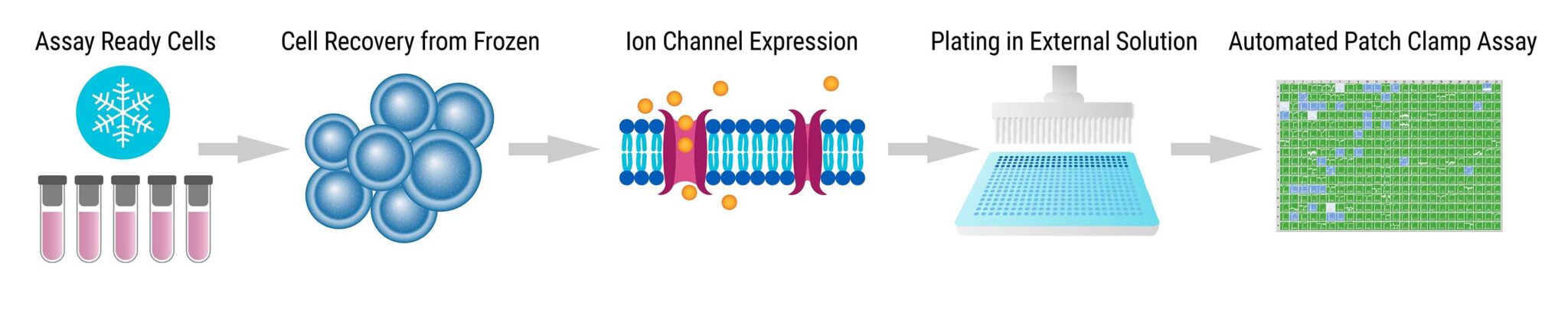
Assay Overview
- Assay Ready Cells were thawed and incubated for 10 hours at 37°C with 5% CO2.
- Cells were grown overnight at 28°C to increase membrane ion channel expression.
- Cells were passaged with 5% trypsin, then diluted to 200,000 cells/mL in external solution and incubated for 60 minutes at 15°C with shaking.
- Automated patch clamp recordings were performed with the Syncropatch 768 PE platform.
Results
Method Validation
MaxCyte® electroporated Assay Ready wild type (WT) Q2/Q3 cells and untransfected CHO-Q3 cells were analyzed by automated patch clamp recording. Average current-voltage relationships were plotted (Figure 1A). Peak current density in WT Q2/Q3 cells is 20-fold higher than in parental CHO-Q3, confirming that the current is dependent on the expression of Q2.
Tetraethylammonium (TEA) ion is a potassium ion channel blocker; different channels exhibit distinct sensitivity profiles to TEA. When WT Q2/Q3 cells were treated with TEA, the concentration response was consistent with previously reported results for wild-type Q2/Q3 heterotetramers and different from the response from either Q2 or Q3 homotetramers (Figure 1B).
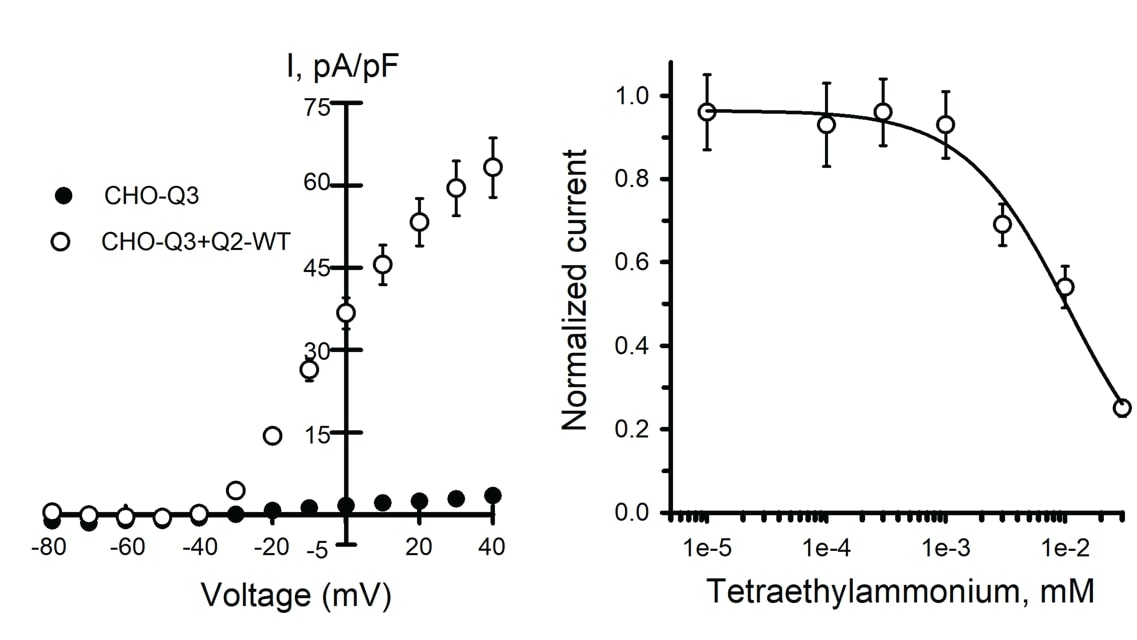
Figure 1. Analysis of Q2/Q3 WT ion channels by automated patch clamp. Mean data +/- SEM (error bars are smaller than some data symbols). A) Average current-voltage relationships from CHO-Q3 cells either non-transfected (n = 94) or transfected with Q2 WT (n = 124) normalized to cell capacitance to calculate the current density (I, pA/pF). B) Concentration-response for TEA block of whole-cell currents recorded from CHO-Q3 transfected with Q2 WT (IC50 = 10.7 +/-4.2 mM, n = 51-82 for each concentration).
The proposed precision therapeutic for KCNQ2-associated epilepsy, retigabine, is a positive allosteric modulator of ion channel activity. Retigabine treatment of Assay Ready WT Q2/Q3 cells caused an increase in peak current density (Figure 2A) and a substantial shift in the voltage-dependence of activation (Figure 2B), consistent with previous reports for Q2/Q3 heterotetramers.
Together, these results demonstrate the validity of automated patch clamp recording of Assay Ready Cells for studying the functional and pharmacological consequences of Q2/Q3 ion channel variants.
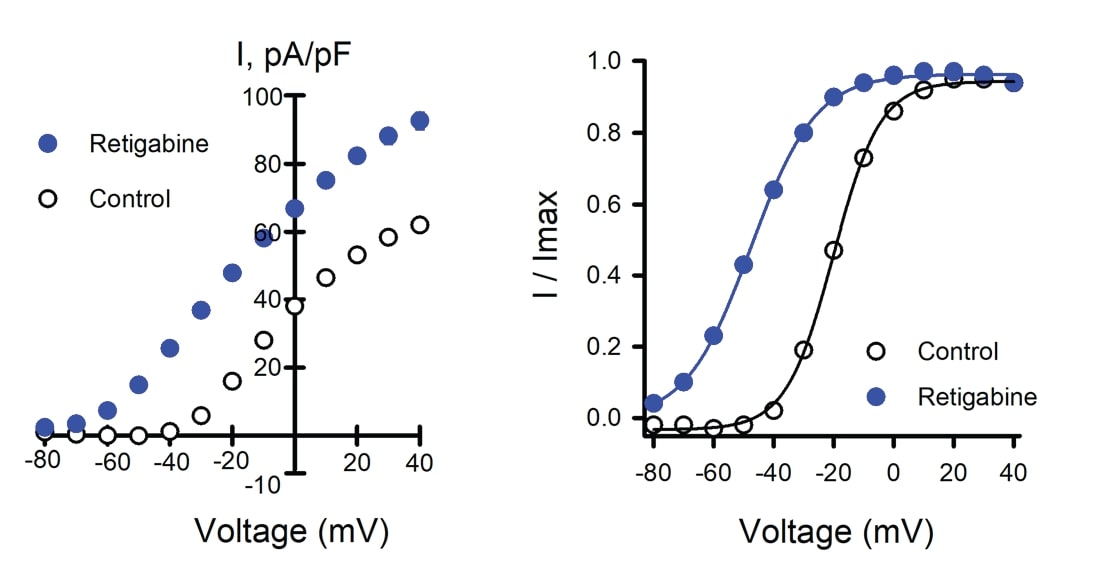
Figure 2. Effects of retigabine on WT Q2/Q3 ion channel. Data shown are mean +/- SEM (error bars are smaller than some data symbols). WT Q2/Q3 cells were treated with a control solution or retigabine. A) Average current-voltage relationships from WT Q2/ Q3 cells treated with control (n = 1086) or retigabine solution (n = 1141). B) Voltage-dependence of activation measured in WT Q2/Q3 cells with control (V1/2 = -18.9 +/-0.2, k = 7.6 +/- 0.1, n = 833) or retigabine solution (V1/2 = -47.7 +/- 0.3, k = 9.9 +/- 0.1, n = 885).
Characterization of Previously Studied Variants
To validate the workflow, the authors compared new data they generated for 18 previously studied variants to published values. Data were normalized to WT channels assayed in parallel, enabling the comparison of results generated by varied experimental approaches in different laboratories. Results from automated patch clamp recording of Assay Ready Cells broadly aligned with previously published data, further validating this strategy for evaluating Q2 variants (Figure 3). Variants studied include those associated with benign familial neonatal epilepsy (BFNE), and developmental and epileptic encephalopathies (DEE).
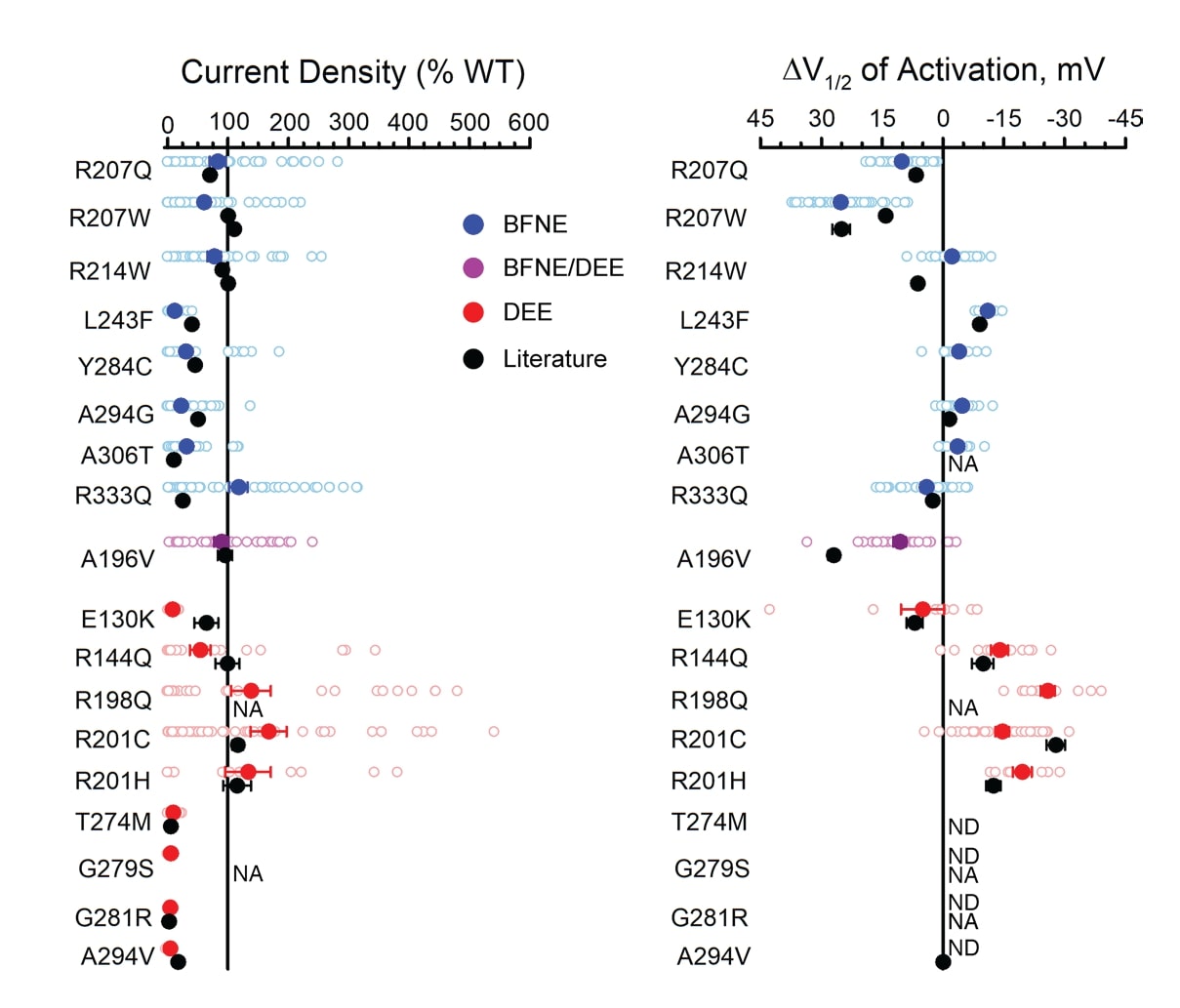
Figure 3. Characteristics of homozygous KCNQ2 variants evaluated by automated patch clamping in Assay Ready Cells are consistent with previous reports. A) Average peak whole-cell currents at +40 mV in Q2 variant Assay Ready Cells as a percent of WT channel measured in parallel. B) Difference in activation V1/2 determined for Q2 variant Assay Ready Cells as a percent of WT (loss-of-function shown as left of the zero line). Individual data points are shown with open circles; filled circles represent mean values. Black circles represent reported mean +/- SEM voltage-clamp data for previously studied variants (some error bars are smaller than the data symbol).
Functional Evaluation of KCNQ2 Population Variants
The authors tested 24 general population variants not associated with epileptic conditions. All of the variants were tested as homozygous channels. Fifteen variants showed different responses to WT and were then tested as heterozygous channels. As expected, none of these non-disease-associated variants showed a loss of function relative to wild type in the clinically relevant heterozygous state.1
Characterization of Disease-Associated KCNQ2 Variants
Having validated the method using multiple controls, the authors examined 39 previously unstudied epilepsy-associated variants. All the variants were screened in both the homozygous and the heterozygous state. Figure 4 summarizes the differences in peak current density and activation V1/2 for heterozygous variants relative to WT. Most of the heterozygous variants had lower peak current density than WT, indicating loss of function. The authors proposed that for the few variants that behaved similarly to WT, the association with epilepsy may be incidental, or the functional significance of the variation might only be apparent in the absence of Q3 or only in neuronal cells.
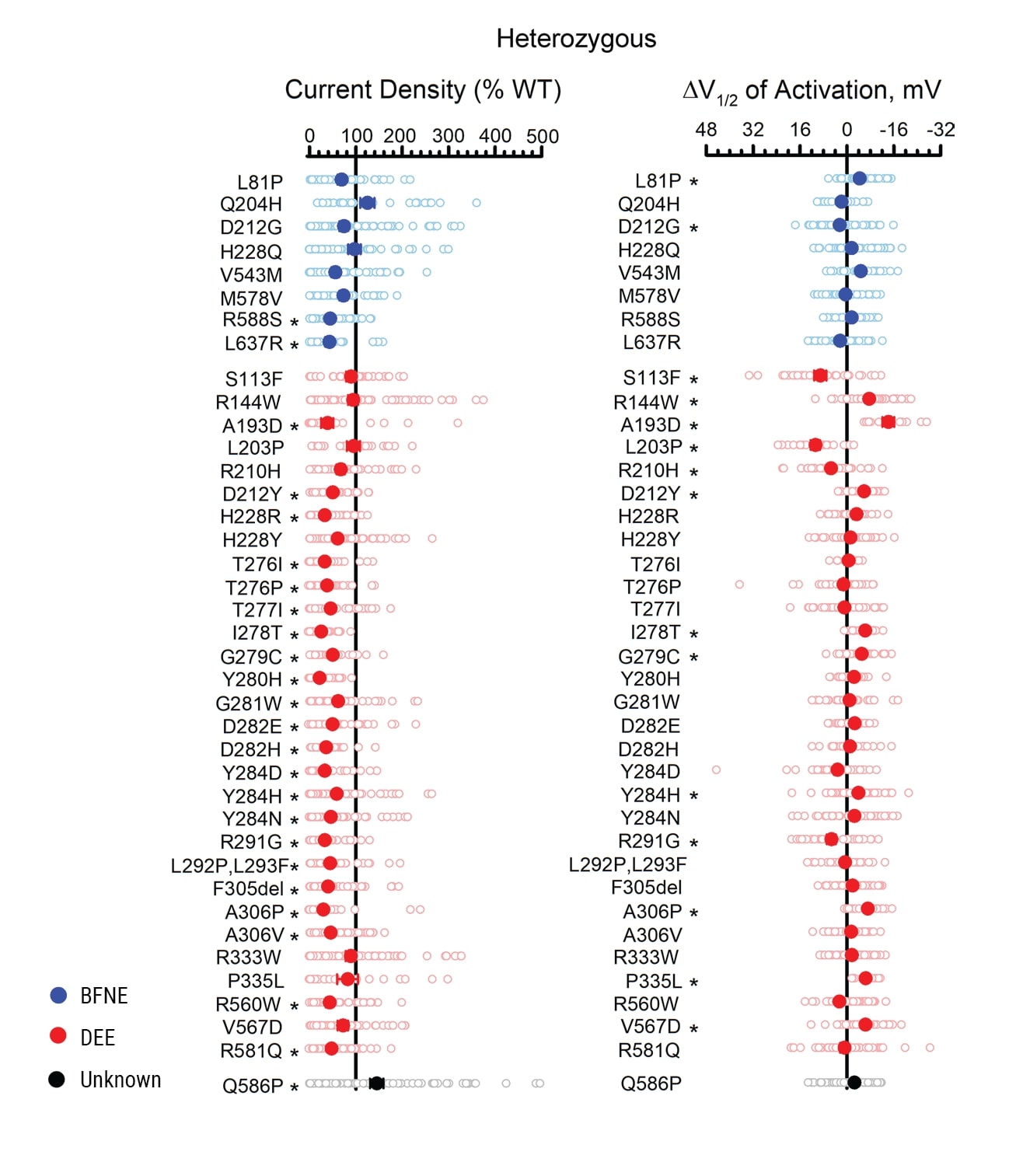
Figure 4. Functional evaluation of epilepsy-associated KCNQ2 variants expressed heterozygously. A) Average whole-cell currents measured at +40 mV for epilepsy-associated Q2 variants relative to the WT channel measured in parallel (n = 19-84). B) Differences in activation V1/2 determined for KCNQ2 variants relative to the WT channel measured in parallel (n = 13-69). Individual data points are presented as open circles, and filled circles represent mean values. Statistical significance was determined using 1-way ANOVA. *P ≤ 0.01.
Variant Responsiveness to Retigabine
The authors examined the retigabine response of heterozygous epilepsy-associated KCNQ2 variants. Figure 5 is a heat map showing the current density of untreated (control) and treated (retigabine) KCNQ2 variants at a physiologically relevant membrane potential expressed as a percentage of untreated WT. Although the heat map illustrates a heterogeneity of responses, ion channel function was restored to at least the WT level with retigabine treatment for all the variants tested.
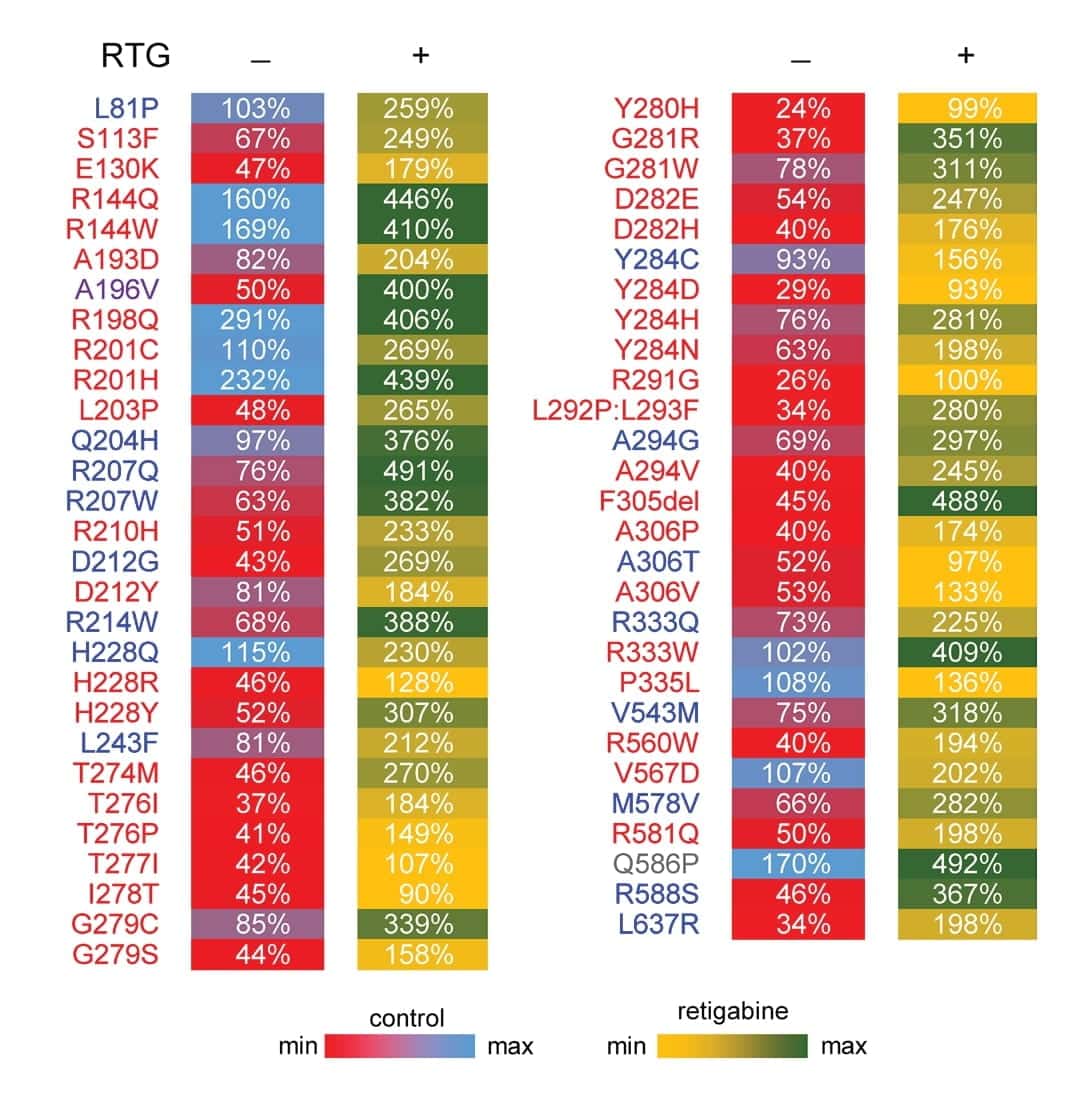
Figure 5. Heterozygous epilepsy-associated KCNQ2 variants show heterogeneity of responses to retigabine. Heat map summarizing current density measured at -20 mV for KCNQ2 variants expressed as a percentage of untreated WT channel current density. Control values (RTG -) are recorded in the absence of retigabine treatment. Retigabine values (RTG +) are measured in the presence of 10 μM retigabine. Each value is colored based on the scale shown.
Conclusions and Future Applications
MaxCyte® electroporation enabled the creation of Assay Ready Cells for method development, validation and characterization of over 150 ion channel variant heterotetramers. This assay workflow can be used for ion channel variant evaluation to deploy emerging precision therapies successfully. The ability to create up to eight Assay Ready Cell banks in a single, quick electroporation process with the MaxCyte ATx® or STxTM ensures that new variants can be screened without delay.
In this study, the authors used CHO-derived CHO-Q3 cells to prepare Assay Ready Cells, but they noted that the functional significance of some variants might only become apparent in neuronal cells. The ability to transfect virtually any cell type with MaxCyte electroporation, including primary neurons and neuronal cell lines, may enable researchers to test that hypothesis in the future.
Combining Assay Ready Cells with automated patch clamp recordings is a rapid, robust method for the high-throughput evaluation of large numbers of ion channel variants. MaxCyte electroporation and Assay Ready Cells provide a flexible approach that can be used for high-throughput analysis of ion channels and other pharmacologically interesting protein classes.
References
- Vanoye CG, Desai RR, Ji Z, Adusumilli S, Jairam N, Ghabra N, Joshi N, Fitch E, Helbig KL, McKnight D, Lindy AS, Zou F, Helbig I, Cooper EC, George AL Jr. Highthroughput evaluation of epilepsy-associated KCNQ2 variants reveals functional and pharmacological heterogeneity. JCI Insight. 2022 Mar 8;7(5):e156314. doi: 10.1172/ jci.insight.156314.
- Dirkx N, Miceli F, Taglialatela M, Weckhuysen S. The Role of Kv7.2 in Neurodevelopment: Insights and Gaps in Our Understanding. Front Physiol. 2020 Oct 28;11:570588. doi: 10.3389/fphys.2020.570588.
This content was adapted and reproduced from Vanoye et al. 2022 under the Creative Commons license Attribution 4.0 International (CC BY 4.0)
* Since the publication of this study, the new ExPERT STx® instrument has been released to include enhanced software, an improved user interface and an integrated touch screen.





