Scientific Brief
MaxCyte Electroporation Enables the Efficient, Scalable Packaging of CRISPR-Cas9 RNPs in Virus-Like Particles
Abstract
Genome editing tools such as CRISPR-Cas9 nucleases hold great promise for treating human disease. To realize that potential, a safe, efficient method for their delivery to target cells will be critical. Viral vectors can be used for efficient, targeted in vivo delivery, but they may come with the risk of off-target editing due to prolonged nuclease expression.
Historically used as vaccines, virus-like particles (VLPs) have recently been investigated for the transient, safe delivery of gene editing tools. VLPs retain the structure and many of the functions of the parental virus, including the ability to package proteins and nucleic acids. Because VLPs cannot self-replicate, VLP manufacturing requires transgene delivery to cultured cells, or the use of purified proteins for in vitro self-assembly.
Here, we demonstrate a rapid, scalable process for manufacturing CRISPR-Cas9 VLPs with high editing activity using MaxCyte’s cGMP-compliant electroporation technology in both adherent and suspension HEK293 cells.
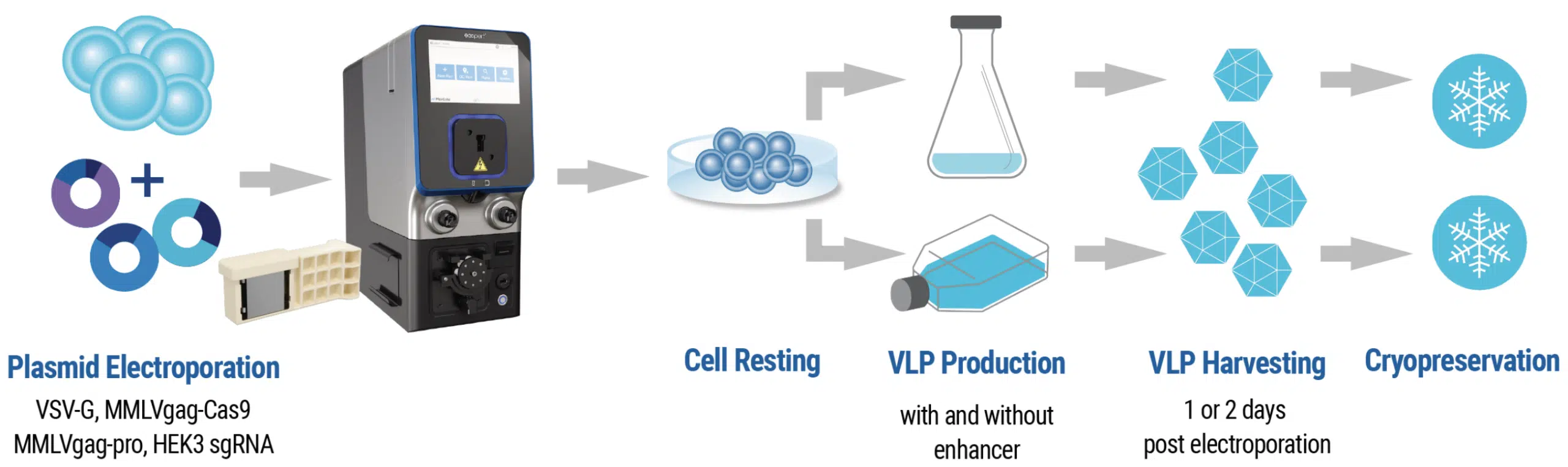
VLP manufacturing workflow
Functional analysis of VLP-Driven editing activity
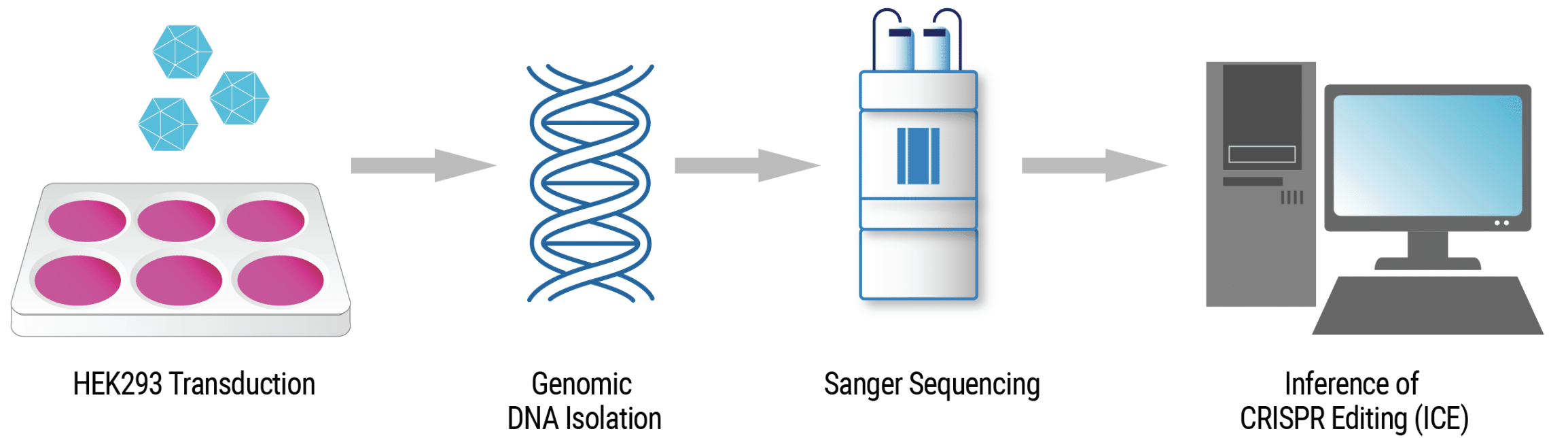
VLP Manufacturing and Analysis Workflow HEK293 cells were harvested and resuspended in MaxCyte electroporation buffer with a four-plasmid mix. Cells were electroporated, rested and then cultured in complete media. VLPs were harvested and cryopreserved after 24–48 hours. The rate of insertions and deletions (% indels) in VLP-transduced HEK293 was determined by Sanger sequencing and analysis with the Synthego ICE Analysis tool.
Results
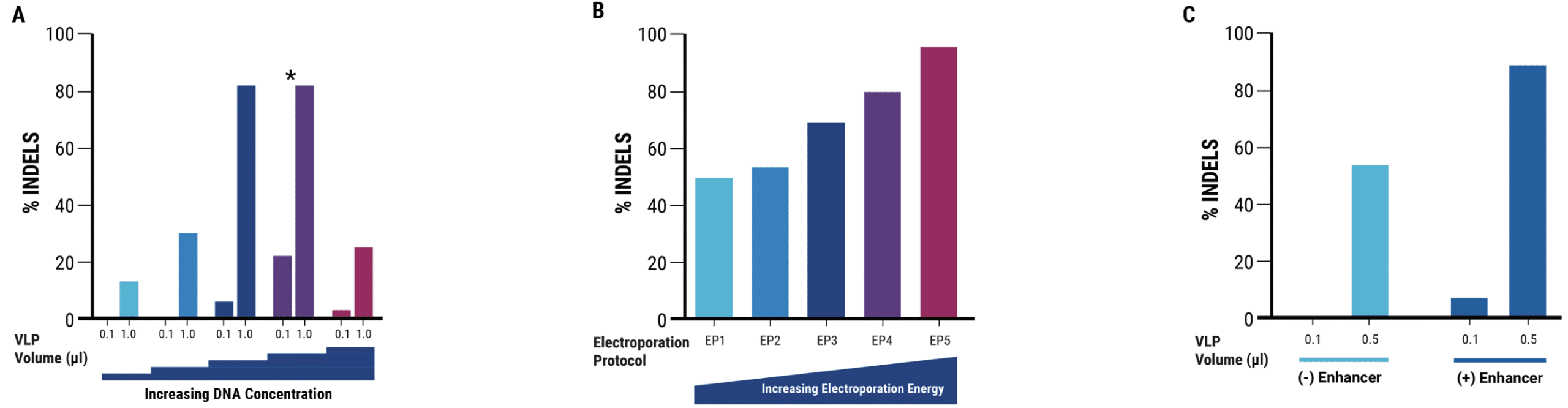
Figure 1. An optimized electroporation workflow yields high VLP editing activity in adherent hek293 cells A) VLP editing activity varied with different plasmid concentrations. The optimal DNA concentration is denoted by *. B) Increasing electroporation energy resulted in higher VLP yields. Electroporation protocol EP4 was used for process optimization experiments and EP5 was used for VLP production. C) Adding a chemical enhancer to electroporated cells increased VLP editing activity.
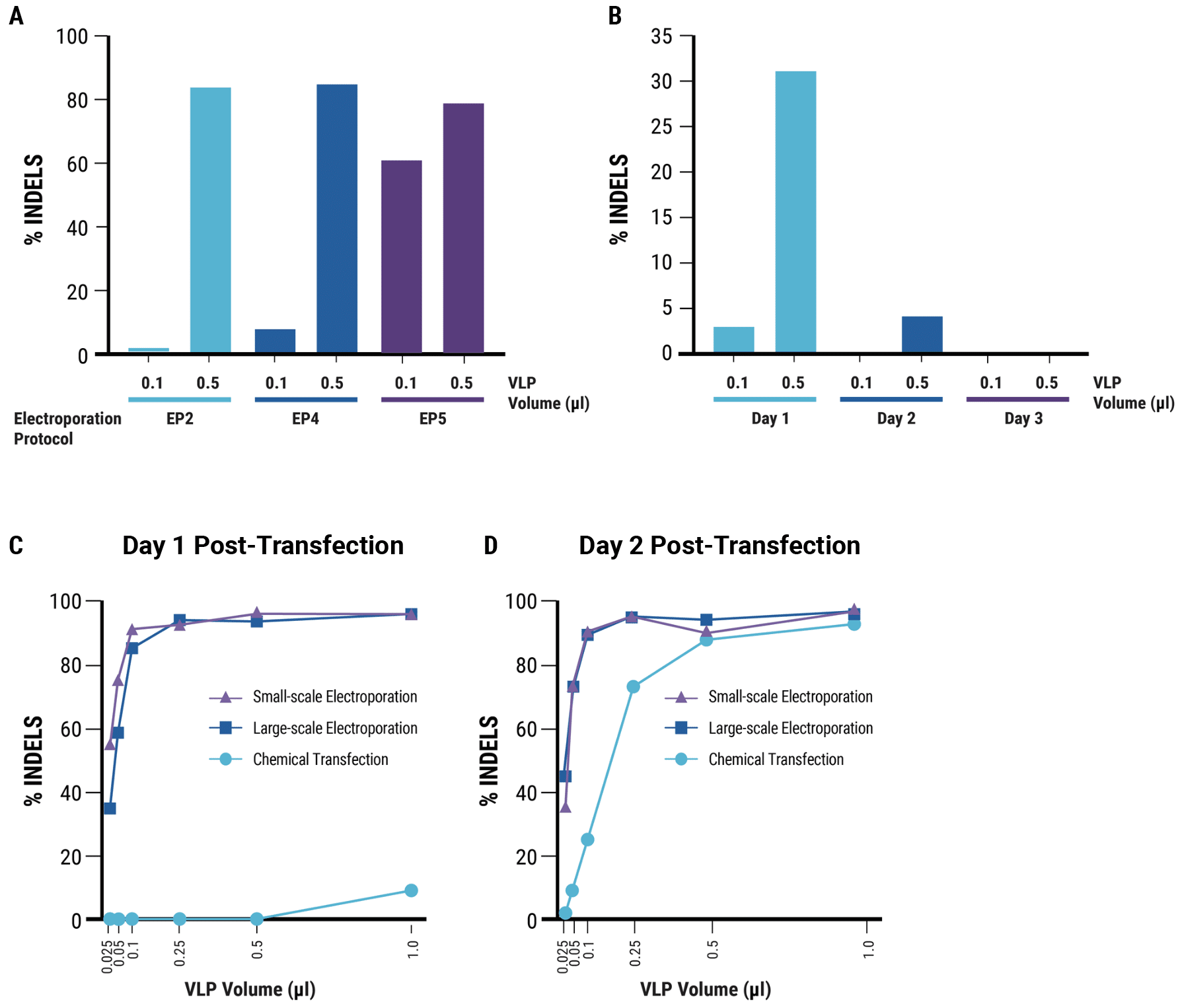
Figure 2. MaxCyte electroporation enables efficient, scalable VLP production in suspension cells A) Suspension HEK293 cells were electroporated with the previously optimized DNA concentration (denoted by * in Figure 1A) and increasing electroporation energy. B) Maximal editing activity was observed with VLPs harvested at day one post electroporation. C) and D) Large-scale Flow Electroporation (1x109 cells) produced similarly high VLP editing efficiency as small-scale static electroporation (1.2x108 cells), using the same optimized conditions. Electroporation produced higher VLP editing efficiency than chemical transfection (1.2x108 cells). Electroporation enables harvesting of functional VLPs on day one post-transfection.
Summary
- Efficient, scalable cGMP-compliant transfection of up to 100 billion cells with MaxCyte’s Flow Electroporation could enable rapid clinical manufacturing of virus-like particles
- MaxCyte electroporation in adherent and suspension HEK293 cells produces high CRISPR-Cas9 VLP editing activity
- Electroporation can reduce manufacturing timelines by enabling day one harvest of highly active VLPs
- MaxCyte electroporation is a simple, time-saving alternative to chemical transfection for VLP production
Related Resources
Have more questions?
Send your question to one of our cell engineering experts.