Application Note
MaxCyte® Enables a cGMP-Compatible Manufacturing Process for Highly Efficient T Cell Engineering
Background
Excitement over recent breakthroughs in autologous cellular therapies has been tempered by the expense of manufacturing with viral gene delivery and concerns over random integration and the safety of viral vectors. MaxCyte offers a non-viral alternative that allows for site-specific delivery of transgenes through homology-directed repair (HDR) and circumvents complex and expensive manufacturing procedures. Although CRISPR-Cas9 technology has led to rapid advances in genetic engineering, the efficiency of knockin (KI) and absolute yield of live KI cells remain challenging, particularly for clinical and therapeutic applications.
In 2020, researchers in the Marson lab demonstrated that an HDR template with Cas9 target sequences (CTS) shuttle more efficiently to the cell nucleus, allowing RNPs to bind and facilitate KI. Even with improved KI, the absolute yield of engineered cells remained limited due to double-stranded DNA (dsDNA) toxicity.1 Now, Shy, et al. have developed an innovative single-stranded construct with two short regions of double-stranded CTS at the sequence ends. This hybrid oligonucleotide allows Cas9 to bind more efficiently while overcoming the problem of dsDNA cytotoxicity. A recent article in Nature Biotechnology demonstrates the integration efficiency of these single stranded Cas9 target sequence (ssCTS), in a range of construct sizes, genetic loci, and cell types.2
MaxCyte electroporation technology enabled the delivery of long ssCTS HDR templates into difficult-to-transfect primary cells. This approach resulted in highly efficient integration (46–62%) of a B cell maturation antigen (BCMA)-CAR sequence into the TRAC locus of T cells. MaxCyte electroporation is clinically validated and scalable facilitating increased production of engineered cells for patient treatment. Finally, the delivery of BCMA-CAR sequences had minimal cell toxicity when CAR T cells were manufactured at clinical scale (>1.5 x 109 CAR+ cells) enabling the commercial production of quality, clinically active, genetically engineered cells for cancer treatment.
Experimental Design

Aim
This groundbreaking study from the Marson lab developed a cGMP-compliant process for non-viral T cell engineering. The experiments featured here demonstrate the ability of the MaxCyte GTxTM to deliver ssCTS HDR templates and CRISP-Cas9 RNPs to modify T cells in clinically relevant amounts.
Method Overview
The summary below outlines a large-scale manufacturing process for engineered CAR T cells. The procedure illustrates the seamless fit of MaxCyte® enabling technology into a clinical-grade T cell manufacturing strategy.
- Harvest – T cells (~ 1 x 108) were harvested from two healthy donors by leukapheresis.
- Activation – T cells were activated using CD3/CD28 DynabeadsTM and cultured with a cocktail of interleukins (IL-7 and IL-15).
- Electroporation – The MaxCyte GTx instrument efficiently co-delivered GenScript® ssCTS HDR templates and CRISPR RNPs. Primary T cells (2 x 108 cells per mL) were electroporated using the R-1000 processing assembly, rested at room temperature for 15 minutes, then cultured for 24 hours.
- Expansion – Twenty-four hours after electroporation (EP) cells were treated with small molecule inhibitor cocktails, either non-homologous end joining inhibitor M3814 or MT (M3814 and histone deacetylase class I/II inhibitor Trichostatin A, TSA). Cells were expanded in a G-REX® culture vessel for 7-10 days.
- Characterization – Engineered cells were isolated and characterized by flow cytometry and immunophenotyping. The functionality of isolated CAR T cells was determined by in vitro killing assay and in vivo survival assays.
Results
cGMP-Compliant Generation of ssCTS HDR Templates
MaxCyte enabled cGMP-compliant delivery of ssCTS required for clinical scale T cell manufacturing. In this study, the ssCTS HDR templates were generated by GenScript. Commercially produced templates consistently showed high KI efficiencies (Figure 1A) and the least cytotoxicity (Figure 1B). This resulted in better total engineered cell counts (Figure 1C). GenScript’s cGMP compliant manufacture of these unique ssCTS constructs and their delivery by MaxCyte electroporation are both integral steps in the clinical scale production pipeline for CAR T cell therapies.
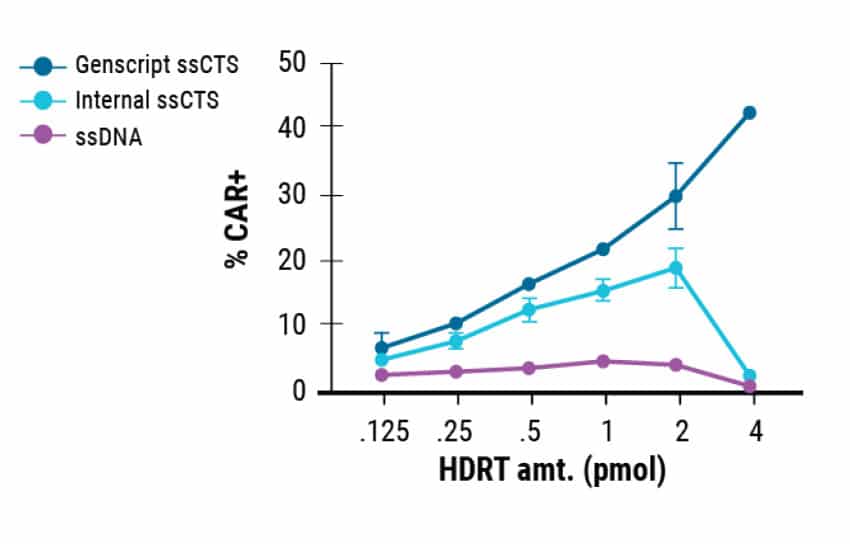
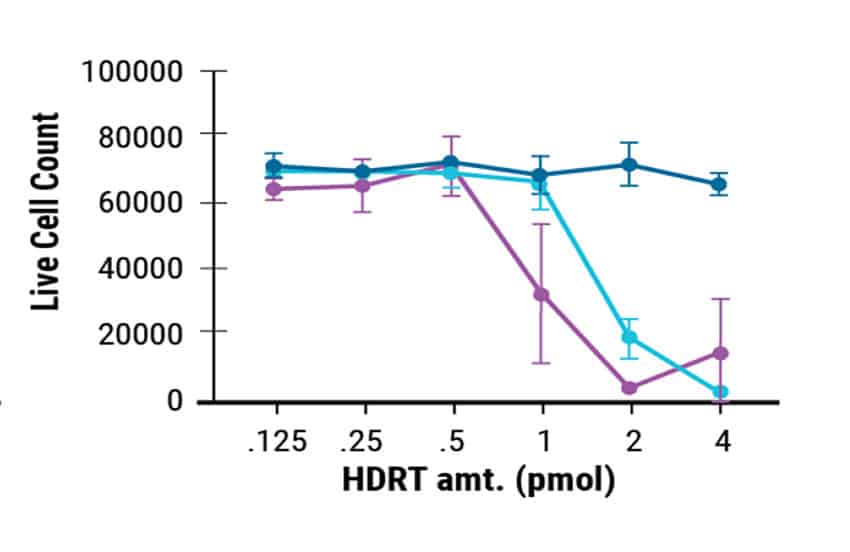
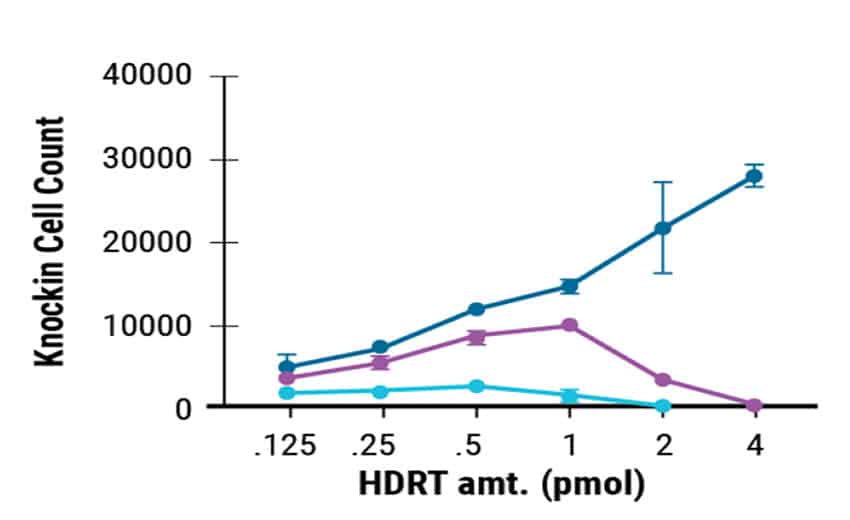
Figure 1. GenScript® Large Scale Production of ssCTS HDR Templates. Comparison of GenScript and internally developed ssCTS constructs from the Marson lab. KI efficiency, cell viability, and total KI cell counts were measured for both constructs with GenScript showing better performance in all parameters tested.
KI Efficiency of Large ssCTS Constructs
To develop an entirely non-viral, cGMP-compliant manufacturing pipeline for CAR T cells, the MaxCyte GTxTM was used for efficient delivery and integration of long ssCTS HDR templates. Engineered cells were modified to express a CAR targeting BCMA, a commonly expressed protein in multiple myeloma and a clinically validated target for immunotherapy.3 Additionally, the BCMA-CAR sequence was designed to integrate into the difficult-to-engineer TRAC locus. The KI efficiencies of the ssCTS templates were impressive with average KI efficiencies of 40.4% on Day 7 and 45.8% on Day 10. The addition of small molecule inhibitors did increase the KI efficiencies to 62% and 60.1% for M3814 and MT respectively (Figure 2B), however, they did not increase the overall yield of engineered cells (Figure 3A) or live cell count (Figure 3B).
Clinical Scale Production of CAR T Cells
MaxCyte electroporation is extremely gentle enabling fast recovery and expansion of sensitive T cells. Encouragingly, the absolute yield of live engineered cells on day 7 was over 5 x 108 cells and increased to over 1.5x109 cells on day 10 (Figure 3A). The MaxCyte GTxTM generated a sufficient number of engineered cells for a full patient dose of an estimated ~1 x 108 CAR+ T cells. After 8 days of expansion, the majority of engineered cells had a T stem cell memory (Tscm) immunophenotype (Figure. 3C and 3D), which is critical for establishing immunological memory and protective immunity.4
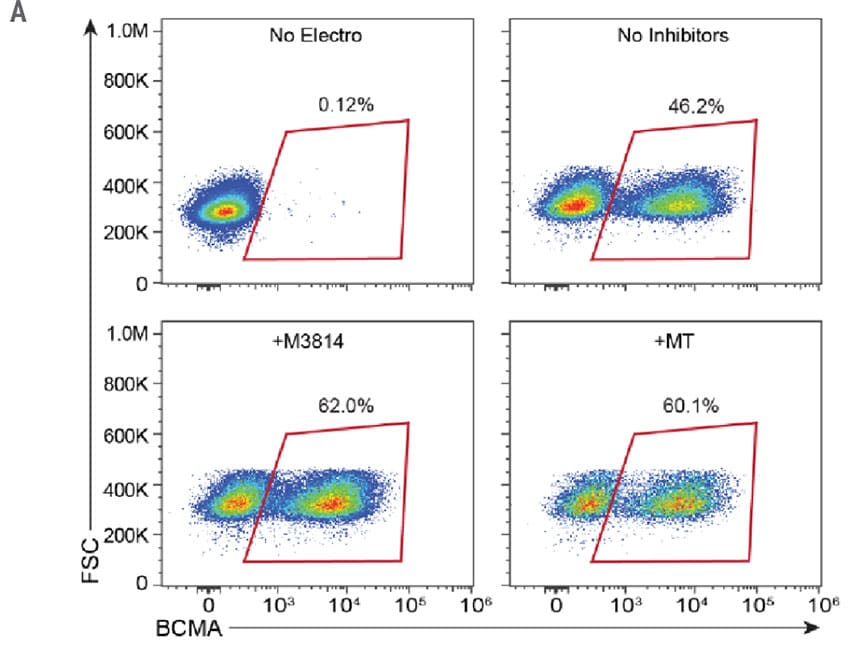
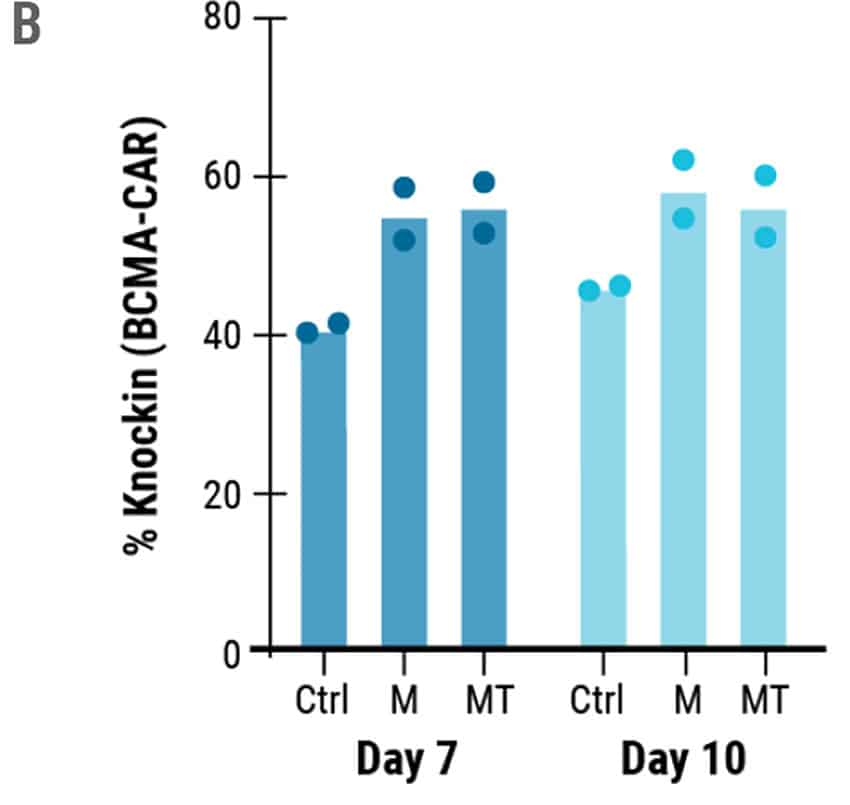
Figure 2. High KI Efficiency at the TRAC Locus Using ssCTS HDR Templates and MaxCyte Electroporation. A) Flow plots demonstrating BCMA-CAR KI efficacies for each condition on 10 day. B) KI rates of the BCMA-CAR on day 7 and 10 for control (no inhibitor), M3814 and M3814+TSA (MT). Dots represent the KI rates for each donor.
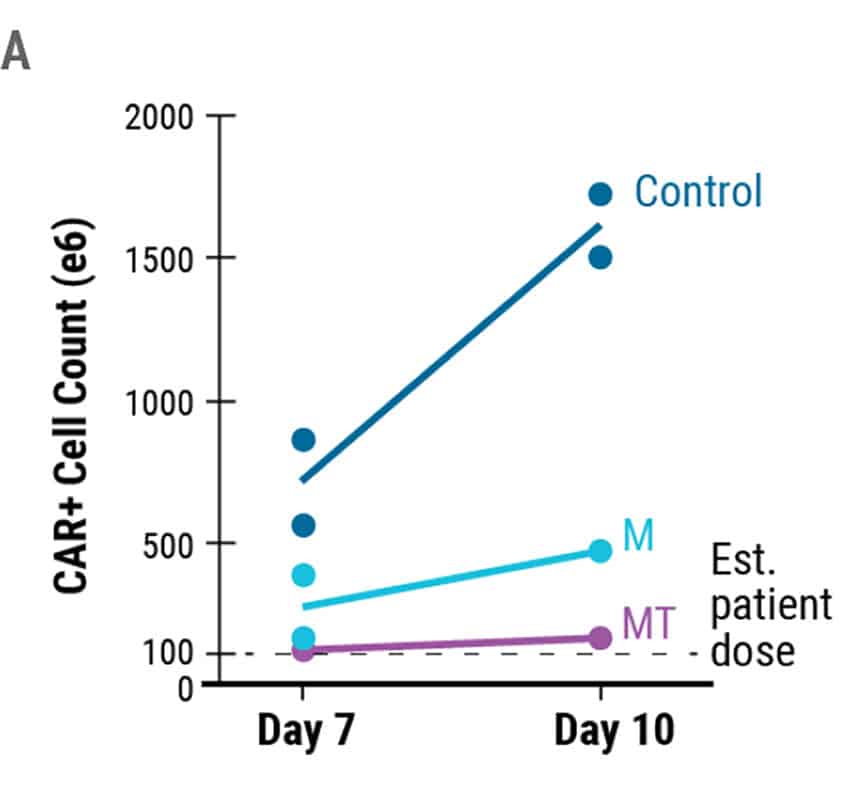

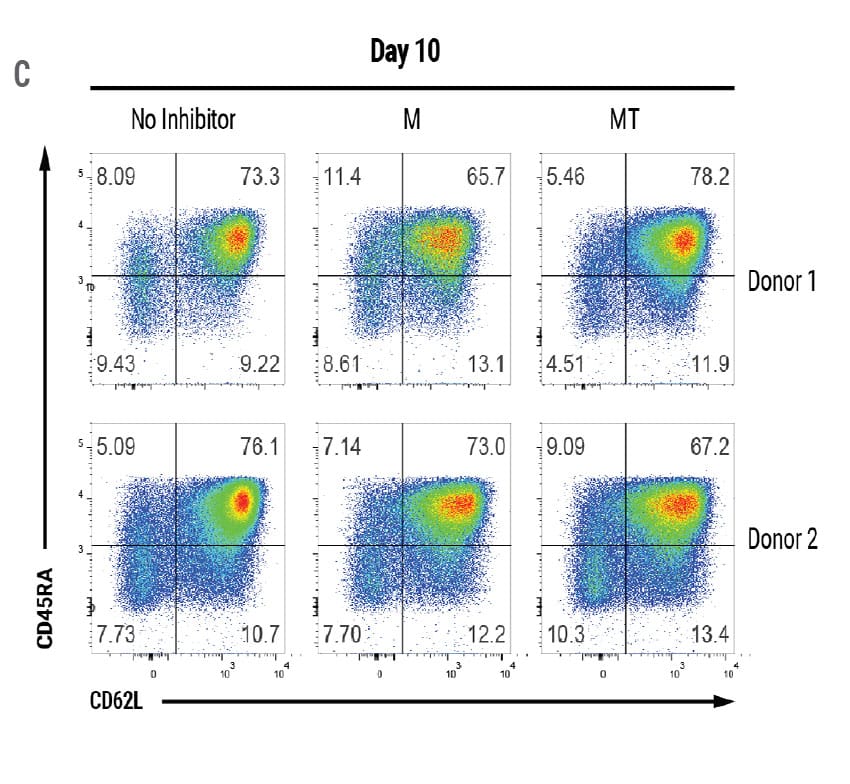

Figure 3. Absolute Cell Count and Phenotypic Analysis of Large-Scale Production of CAR T Cells. A) The total number of CAR+ T cells on days 7 and 10 of the cGMP manufacturing protocol. The dotted line represents an estimated patient dose (1x108 cells). B) Live cell count was performed 7 and 10 days post activation. Dots represent live cell count for each donor. C) Cell immunophenotypes were confirmed through flow cytometric analysis of the indicated cell surface markers 8 days after EP. D) Flow plots demonstrating the presence of T stem cell memory cell (Tscm) based on CD45RA/CD62L expression and confirmed with additional markers such as CD45RA+CD62L+CD45RO-CCR7+CD95+. M = M3814, MT = M3814 + TSA, Tscm = T stem cell memory, Tcm = T central memory, Tm = T effector memory, Teff = T effector.
Functional CAR T Cells Kill Tumor Cells In Vitro and In Vivo
To determine if engineered T cells could target and kill cancer cells, an in vitro killing assay was performed. Engineered CAR T cells or unmodified T cells from the same donors were co-cultured with cancer cells expressing BCMA and cancer cell viability was measured (Figure 4A). Engineered CAR T cells were highly potent and demonstrated efficient killing against MM1s myeloma cell lines compared to controls. Tumors were established in NSG mice using MM1s cells. Engineered CAR T cells efficiently reduced the tumor burden resulting in enhanced survival (Figure 4B).
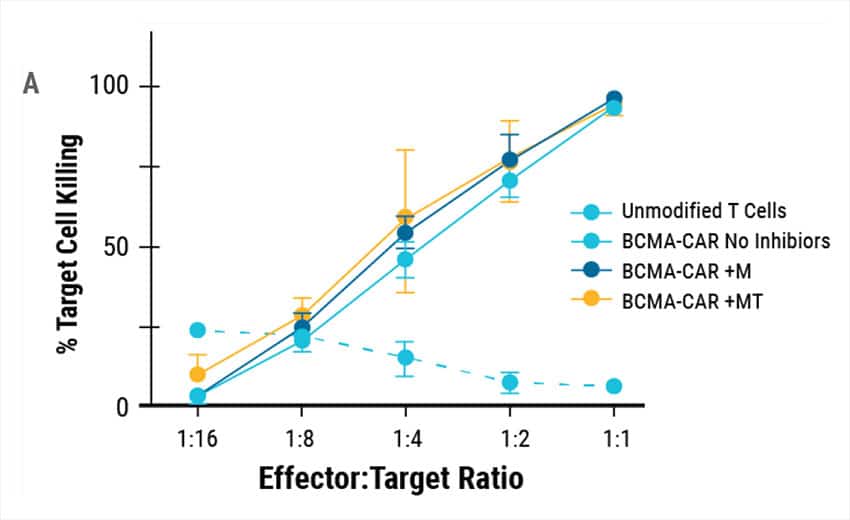
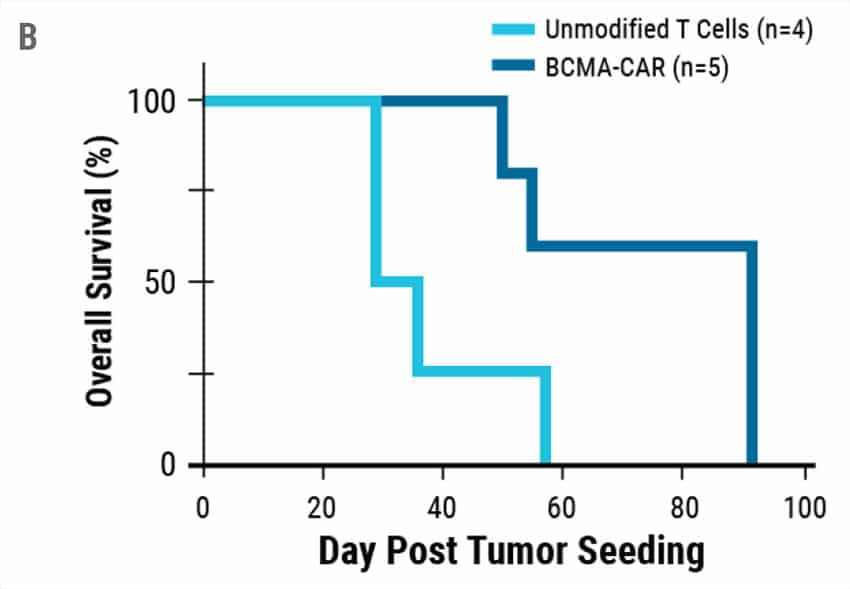
Figure 4. Cytotoxic Activity of Engineered CAR T. A) BCMA expressing multiple myeloma cell lines were effectively targeted and killed by engineered CAR T cells. At effector to target ratios of 1:1 over 90% target killing was achieved. B) Overall survival in tumor-bearing mice when treated with engineered CAR T cells or unmodified T cells from the same donor.
Conclusion and Future Applications
In summary, a fully cGMP-complaint, non-viral manufacturing pipeline for engineering highly specific and potent CAR T cells was established. MaxCyte enabled the production of engineered T cells at a clinical scale through highly efficient delivery of ssCTS constructs resulting in KI integration efficiencies of up to 46–62% at the challenging TRAC locus.
The study presented here is an important step toward improving HDR-associated CRISPR gene editing and showcases a new approach to the large-scale manufacturing of engineered cells. MaxCyte’s cGMP-compliant Electroporation technologies streamline the development of new therapies by reducing the complexity of scale-up and is a strong foundation for the advancement of genetically engineered cells into the clinic.
References
- Nguyen DN, Roth TL, Li PJ, et al. Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency. Nat Biotechnol.2020;38(1):44-49. doi:10.1038/s41587-019-0325-6
- Shy BR, Vykunta VS, Ha A, et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails [published online ahead of print, 2022 Aug 25]. Nat Biotechnol. 2022;10.1038/s41587-022-01418-8. doi:10.1038/s41587-022-01418-8
- Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384(8):705- 716. doi:10.1056/NEJMoa2024850
- Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med. 2017;23(1):18-27. doi:10.1038/nm.4241






