2024 ASGCT Presentation
Streamlining the CRISPR Editing Process in an eTIL Cell Therapy
American Society for Gene and Cell Therapy annual meeting
Baltimore, Maryland, USA
May 9, 2024
Ben Askin presents KSQ Therapeutics' work developing a scalable cGMP process for their engineered tumor-infiltrating lymphocyte (eTIL™) cell therapy.
Watch the ASGCT presentation

Presenter

Have more questions?
Send your question to one of our cell engineering experts.
Popular resources
Transcript
This is the work that KSQ performed in collaboration with MaxCyte to streamline our CRISPR editing process for our eTIL cell therapy. So despite 100 years of progress in oncology, cancer remains the highest death, cause of death for those under the age of 65. This is largely driven by solid tumor indications. Approximately 90% of cancer cases are solid tumors, and nearly 50% of all cancer cases come from four solid tumor indications; so are cancer deaths. And, its KSQ's mission to use new tools, like CRISPR and the knowledge gained from the Human Genome Project, to drive progress forward in oncology again.
So, there's two biological traits that have made solid tumors challenging to treat. Tumor antigen heterogeneity, which essentially means that each patient has their own unique cancer. And not only that, even within the tumor, there is differences in antigen presentation, cancer cell to cancer cell. Additionally, there's the tumor microenvironment, which is potently immunosuppressive. Tumors recruit cells like Tregs, secrete cytokines like IL-10 and deplete the immune cells that have penetrated the tumor of nutrients.
Additionally, you have the extracellular matrix, which even makes it difficult for the immune cells to enter. All these things make it difficult for the tumor to be targeted, reached and killed. But, KSQ's eTIL resolves these issues. eTILs are personalized, meaning they come from the patient's tumor, and they have the ability to recognize that patient's unique cancer antigens. They're polyclonal, which means that our manufacturing process retains that ability to identify those different anitigen presentation within the cancer cells. And, our eTIL are enhanced with CRISPR knockouts to overcome immunosuppressive tumor microenvironment.
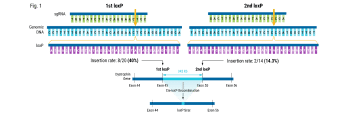
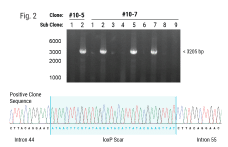
So, we performed a genome-wide screen to identify the top knockout targets that would drive T-cell infiltration into solid tumors in mouse models. And out of those screens, REGNASE-1, which is a modulator of stem central memory cell formation in tumors, and SOCS1, which is a negative regulator of cytokine production that stimulates T cell proliferation, were hits on our screen.
Additionally, PD-1, which we all know, showed a pie on our screen, which gave us confidence that the model was mapping to the biology. We also performed a combo screen which showed, again, that SOCS1 and REGNASE-1 were good combo partners and had synergistic effects when paired together.
And, this is the stuff that makes us really excited. It's one thing to see it on a dot on a graph, and it's another thing to see our eTIL therapies melting away tumors in a PD-1 refractory mouse model.
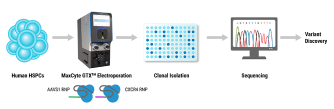
Part of the reason why we're so excited to be entering the clinic this year with KSQ-001 EX and why we're confident is because of the work that we've performed with MaxCyte. It's really two editing experts working together to optimize eTIL. We have robust editing efficiency in eTIL. Specifically, this is data from 001 EX. And, we don't have to sacrifice cell health to achieve that. And that's because, we've done the development work to select the correct guide, the correct ratio of Cas9 to guide, the electroporation program's energy and duration, and handling the cells prior to electrification. But most importantly, post electrification when the cells are at their most sensitive. And that's, been something that MaxCyte has supported us hand-in-hand where they've had knowledgeable site reps like our SME, Joan, who has been with us if we have any experimental design questions or we need help with troubleshooting an issue that we encounter.
And, Joan also provides hands-on training for our new staff so that they know how to handle those cells when they're sensitive, and, also, the old operators like me to make sure that we haven't forgotten. And then, the platform also enabled a scaled-down model, which is also important for when you're working with cell therapy because your celluloids are variable. You don't know what the patient has gone through in terms of treatments, and, yeah, just sometimes, the cells aren't that healthy. And, so you don't always expect, you can't always expect, how much you're going to get for a given experiment, and you're dealing with a lot of conditions that you're working with. And, it's really helpful to be able to work with a scaled-down model and then know that, once you move up to the clinical scale that you're moving to, that those results that you use in the scaled-up model are going to be reflected in the large-scale model.
So, some of the development work that we performed last year was to simplify our electroporation and cell-editing workflow. We had a very narrow cell concentration range that we were working with, and that meant that we had to have a high volume range to account for that variability in the cell yield during when we're engineering the electroporation process.
And, you know, what I'm showing here is volume A to B is like the lowest cell yield, and that, in turn, the lowest volume to keep in that narrow cell concentration range. And then if you move all the way down to a high cell yield, you would need up to three CL1.1s. So, what we wanted to move to was expanding our cell concentration range, and this would allow us to, in turn, shrink down the volume range that we had going into our electroporation process and allow us to work with just a single CL1.1.
So, to study this we performed, yeah, well two studies. The first was a small-scale titration where we were working with MaxCyte's small-scale processing assemblies, and we looked at expanding the cell concentration range above and below what we had previously had as a limit. And, we were just looking for trends in the data to see if there was any effects in that extended cell concentration range.
And then the second study, we moved up to our CL1.1, which is what we were trying to use for our clinical scale and had the targeted cell concentration limit for what we would need to achieve for moving into a single CL1.1, and tested that against the old cell concentration limits.
And, this is the results from study one. I'm not going to give you the actual cell concentrations that we use, but, you can see in the data that there aren't any trends in the reduction in cell health. And, for percent editing, that's the same except for perhaps the lowest cell concentration range that we looked at. That one condition has a little bit more variability, but, ultimately, this isn't, this was one level past what we would need to move into for a single CL1.1 process to be enabled. So, we felt confident moving into the second study, and, again, the results confirm that we had good cell health and good percent editing for the old cell concentration limit compared to the new.
Yeah. And so, the study results made us confident in moving to our new, expanded cell concentration range and, in turn, simplifying our process down to a single CL1.1. And not only does this say processing time, which is important, again, because those cells are so vulnerable after electroporation, but it also saves in COGS and, maybe even more importantly than all those other factors, the manufacturing complexity. Because in cell therapy, you only get, well, I shouldn't say only, but most of the time, you only get one shot at treating the patient. And, we want to make sure that we give our manufacturing operators the best chance at treating these patients. And, you know, they're giving us a part of their body, and we want to make sure we do our due diligence to do the best we can with that. And, it's our hope in PD that we've played a small part in helping the patients that have been diagnosed with the indications we're going after during the time I've been talking today.