2024 ISCT Presentation
Gene Editing in Hematopoietic Stem Cells for Monogenic Blood Cell Diseases
International Society for Cell and Gene Therapy annual meeting
Vancouver, Canada
May 31, 2024
Donald B Kohn, MD, and Zulema Romero Garcia, PhD, present their research advances in gene-editing technologies to expand the horizons for treating monogenic blood cell diseases
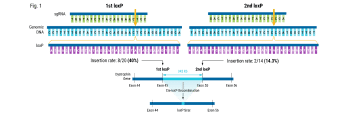
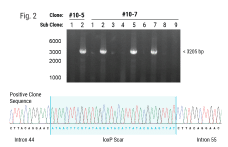
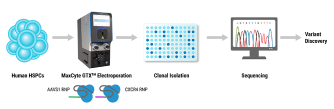
Hematopoietic stem cells (HSCs) are responsible for life-long production of all blood cells and can be collected, manipulated ex vivo for gene addition or gene editing, and re-engrafted by intravenous infusion. Gene therapy using lentiviral vectors has successfully achieved clinical improvements for more than a dozen hematological disorders, including primary immune deficiencies (PID), hemoglobinopathies, lysosomal storage and leukodystrophy disorders. While the safety profile of lentiviral vectors in HSCs has been good, lentiviral vectors lack precision in integration and may not recapitulate precise expression patterns needed for physiologically regulated genes. Gene editing of endogenous loci has the potential for precise insertion and physiologically regulated expression. A collaboration between investigators at UC Berkeley, UC San Francisco and UC Los Angeles has led to a clinical trial that will use Cas9-mediated homology-directed repair with a single-stranded oligonucleotide donor to revert the sickle cell disease (SCD)-causing mutation in HBB. Preclinical, investigational-new-drug-enabling activities involved translation of methods for gene editing in SCD patient HSCs to good-manufacturing-practices-compatible processes with subsequent cryopreservation formulation and release testing. Unlike SCD, where all patients have the identical single nucleotide mutation, most human genetic diseases have heterogeneous mutations across the affected gene in different patients. We are approaching two primary immune deficiencies (X-linked Hyper IgM Syndrome {X-HIM} and X-linked Agammaglobulinemia {XLA}) using Cas9-mediated site-specific insertion of minigene cDNA cassettes into the 5’ end of their respective genes (CD40L and BTK). Both of these genes require precise, physiological expression for safe and effective humoral immune reconstitution and the inserted normal copy of the gene will be under transcription control of the endogenous gene regulatory elements. The thresholds of engrafted gene-edited HSCs needed for therapeutic effects on antibody production are expected to be relatively low (5-20%), based on BMT studies in murine disease models of X-HIM and XLA. We have demonstrated restoration of normal expression patterns of each of these genes in patient-derived cells and in murine models, with development of appropriate B cell functions. We are applying adenine base editing to revert a recurrent stop codon in the CD3δ gene responsible for a genotype of severe combined immune deficiency (SCID) in an ethnic population. CD3δ SCID patient bone marrow CD34+ HSPC were treated with the adenine base editor to correct the CD3D mutation and had restored capacity to produce T lymphocytes in an in vitro artificial thymic organoid system. These advances in gene editing technologies are expanding the horizons for treating monogenic blood cell diseases.
Watch the ISCT presentation

Presenters


Have more questions?
Send your question to one of our cell engineering experts.