Combining CRISPR & Transposon-Based Technologies for Improved sdAb-Based CAR T Therapies
Cell & Gene Therapy Insights
Webinar
June 17, 2025
Watch how a streamlined, one-step process for the generation of universal CAR T cells can reduce costs and accelerate clinical translation, while maintaining potency and durability in hematological malignancy models
In this session from Cell and Gene Therapy Insights, Begoña Díez Cabezas and Juan R Rodriguez-Madoz present Combining CRISPR & Transposon-Based Technologies for Improved sdAb-Based CAR T Therapies. They discuss how viral-free transposon systems, in this case transfected with the MaxCyte ExPERT™ platform, can improve the efficiency and scalability of CAR T manufacturing.
Key topics covered in this session
- CRISPR technologies provide useful tools to generated TCRKO/HLA-IKO CAR T cells for allogeneic approaches.
- The combination of state-of-the-art technologies (transposons, CRISPR, CAR designs, etc.) allow the production of safe and efficient genome-edited CAR T cells, reducing manufacturing costs.
- Pre-clinical evidence demonstrates comparable efficacy between transposon-based CAR T cells and conventional CAR T cells in vivo and in vitro.
- Universal CAR T cells generated by the combination of CRISPR and transposon-based technologies can be produced at GMP level, allowing their evaluation in clinical trials.
Watch more on combining CRISPR and transposon technologies

Presenters


Have more questions?
Send your question to one of our cell engineering experts.
Related resources
Transcript
Jokūbas Leikauskas (moderator): Hello, and a very warm welcome to today's Cell and Gene Therapy Insights webinar titled Combining CRISPR and Transposon-Based Technologies for Improved SD Antibody-Based CAR T Therapies. I'm an editor at BioInsights, and joining me today are Begoña Diez Cabezas and Juan Roberto Rodriguez-Madoz, who will demonstrate key preclinical findings showcasing the comparable in vitro and in vivo anti-tumor efficacy of transposon-based CAR T cells relative to conventional CAR T therapies.
After the presentation, we'll have a live Q&A session. We invite our audience to pose their questions to Begoña and Juan using the “ask a question” box at the bottom of your screen and we'll try to get to it during the session. I would also like to draw your attention to the resources on the righthand side of the screen where you can find more information on the topics covered today.
Next, I would like to introduce our presenters. In 2015, Begoña Diez Cabezas obtained the doctorate in genetics and cell biology from Universidad Autómona from Madrid with the qualification of outstanding cum laude and the European doctorate mention. Since October 2017, she has continued her work as a postdoctoral scientist in the Biomedical Innovation Department at CIEMAT, focused on the production of different gene and cell therapy drugs in the GMP Clinistem facility, first as a production supervisor and since 2022 as a production manager. She has also been collaborating in the development of new CAR T therapies for the treatment of different neoplasms.
Juan Rodriguez-Madoz, obtained his PhD degree in biology in 2005 at the University of Navarra. After his postdoctoral studies, he joined the Hemato-Oncology Program at the Cima Universidad de Navarra, where he is the principal investigator of the Immune Therapies Laboratory. His research line is focused on the development of improved CAR T therapies for hematological malignancies by combining state-of-the-art genome editing and non-viral technologies. His laboratory is also focused on the understanding of molecular mechanisms governing CAR T cell function, combining multiomic approaches at the single-cell level of spatial transcriptomics.
So without further ado, I'll hand over to Begoña to kick us off.
Begoña Diez Cabezas: Thanks a lot for the introduction. I'm just going to present the first part that is regarding the design of the TranspoCART protocol. And just to let you know what are the advantages of the transposon system and why we chose that for the production of a CAR construct is because this construct has a safer integration profile than antiviral vectors because it has a more random profile integration. Also, it drives a high and a stable gene expression. And the most important part for us is that these kinds of vectors present a simpler manufacturing process, and this can lower the production cost of the therapies for patients.
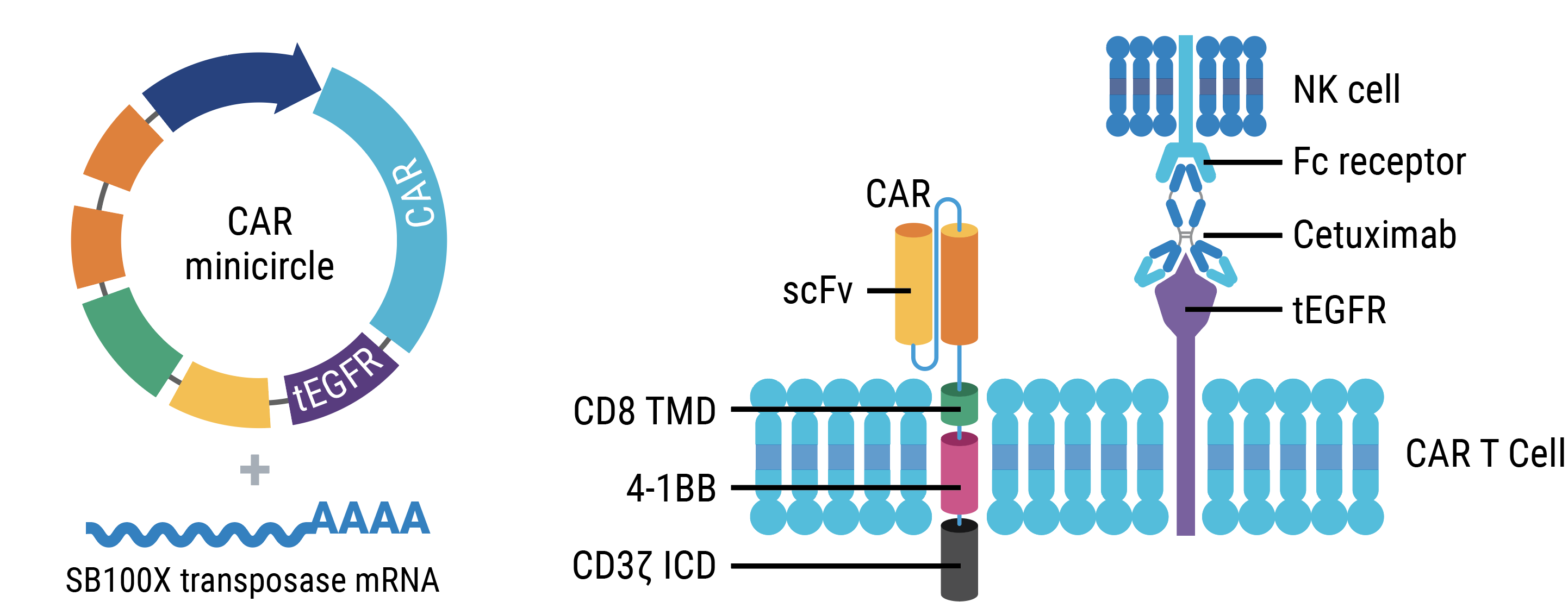
Just to remind you how the system works, it presents two different molecules. On one side is the transposon vector that carries the CAR construct that is flanked with IR sequences, and the other molecule is the transposase. This transposase recognizes IR molecules, and that's a simple cut-and-paste mechanism, which leaves the incorporation in the genome.
So taking this into account, we designed this vector, that you can see in the slide, in which our CAR construct is driven by an EF1α promoter. And we have a safety switch mechanism, which is the EGFR-truncated version [EGFRt] that can be recognized by the antibody cetuximab. Regarding our CAR construct is a second-generation CAR that presents the scFv from the Yescarta and Kymriah, already approved ATMPs. We chose this scFv because we want to demonstrate that using a different kind of protocol, we can produce the same kind of results that the previous already approved ATMPs.
So with this, we designed this protocol, that you can see, in which we start for with a CD4 and CD8 isolation at day zero. And when we activate the cells, two days after, we electroporate the cells with the MaxCyte system using the CAR construct as a minicircle and the transposase as mRNA. And then we left these cells expanding for 11 days in which we only did a refreshment of the cytokines at the middle of the expansion period.
As you can see in the slide, in the top part, we have an increment in the number of cells when we start using samples from healthy donors or patients that are completely equal. And also regarding the transfection, we have good transfection efficiencies around 50%—doesn't matter if we start from healthy donor samples or patient samples to carry on the protocol, we have the same transfection. Regarding the cytotoxicity (that is in the bottom part, on the right) you can see that the CAR T cells that come from patients or healthy donors are as equal as cytotoxic and are more cytotoxic than control cells that are not transfused with a transposon.
To evaluate the kinetics of minicircle and transposase stability inside of our cells during the production protocol, we developed these two studies. The first one is in the left side of the screen, and you can see that we just evaluate by qPCR non-integrated minicircle during the production period. So you can see that at the beginning of the production period, of course, we have a lot of transposons there, but then at the end of the expansion, these transposon disappear. The same happened when we studied the kinetics of the transposase by Western blot, as you can see in the right part of the screen.
With all of this, we do also, of course, in vivo cytotoxicity studies in NSG mice with a protocol that you can see in the top part of the slide. And you can see in the bottom right of your screen that the survival of these animals is improved when we just use the healthy donor samples that are in orange and also samples from patients with different diseases. And the most important part for increasing survival is the same as with one when we use our lentiviral vectors instead of CAR transposon.
Another thing that we studied was if the safety switch that we include in our construct, the truncated version of the EGFR receptor, is working. And with it the same, we developed the protocol that you can see in the top part of your screen. When we just evaluate by blood transfusion the presence of human lymphocytes in the cells, we can see that when we apply the cetuximab that these CAR T cells disappear from the animals.
One of the main things that we have to take into account when we develop an ATMP is the safety of this product, and we just studied the insertion site analysis in collaboration with Zoltan Ivics’ laboratory. And the results were the same, that has been already published, in which the insertion preferential site of this transposon is in TA sites of the genome. So it's more random than lentiviral vectors. And one of the most important parts is that independently of the facility in which these products are developed, we have the same integration pattern.
With all of these, we just do a pre-validation in both facilities starting from the same material. As you can see on the screen, we have exactly the same results, and all the release criteria were fulfilled for this ATMP.
With this, we are now finally running a clinical trial—the design is on the screen now—and you can see that is a 3+3 classical design. We have concluded the first escalation dose, that is 1 million of CAR T per kilogram of weight in the patients. The first three patients have been treated, they are performing well, and we can detect the TranspoCART expression in all of them.
To sum up this part of the presentation, we can say that TranspoCART cells can be produced, either for unhealthy donor or patient samples; that these cells are functional in vivo and in vitro, and the TranspoCARTs present a safer integration profile than lentiviral-based CAR T cells. So we can conclude that the GMP manufacturing of these cells is feasible, and the therapeutic products are functional. And now we are evaluating this in a clinical trial.
And with this, I give the presentation to Juan, who is going to present the next slides.
Juan Roberto Rodriguez-Madoz,: Thank you very much. So, as Begoña mentioned, we have developed and optimized this next protocol for the integration of GMP-compatible, transposon-based CAR T cells that are actually under clinical evaluation. However, for some of the diseases or the applications of these transposon-based CAR T cells, the use of those approaches has an issue. Because, as an example in patients with acute myeloid leukemia [AML], once they relapse, the median overall survival is very short, less than eight months. So sometimes we don't have time for the production of CAR T cells after relapse.
This is why, for these new therapeutic approaches, probably improved CAR T cells and of the use of allogeneic treatments will be more suitable since they will be ready to use. In that sense, as a proof of concept, we have worked in the lab developing improved CAR T cells, started in CD33. That is one of the main targets that is frequently expressed in the AML blasts, and we have optimized, then, as you can see in the bottom of the slide, ending up with a CAR that is very active from the ones that we have already tested.
Moreover, on top of that the generation of autologous CAR T cells could be an issue. We already know that the generation of CAR T cells from AML patients produce CAR T cells that are are functional, but they are a little bit dysfunctional compared with the CAR T cell from healthy donors, indicating that the use of allogeneic CAR T cells will be better for the treatment of these patients.
So to do that, the allogeneic CAR T cells have several advantages. As I already mentioned, the availability, they're ready to use the, since we can have them produced in the lab and properly stored for the infusion to the patient. Also we can have a better quality of the starting material. We can better optimize the standardization of the manufacturing process, and [there’s ]also a decreased cost, since the production of just one CAR T cell product could be used for several patients.
However, the use of allogeneic CAR T cells also has several disadvantages. One of them is the immune rejection. Because we are using CAR T cell from a donor, the patients can't recognize these CAR T cells and eliminate them. But also, and more importantly, they graft surgical disease. Since we are using T cells, the presence of the endogenous TCR from the healthy donor can recognize something in the patient and develop this graft versus host disease.
To avoid that, one of the things we can use is genome editing technologies to deplete the key molecules involved in those processes. For that, we can use many different strategies, but we are going to focus on the combination of the CRISPR-Cas technologies to knock out the expression of these two molecules, HLA-I that will avoid the immune recognition or immune rejection by the patient, and also the endogenous TCR from the T cell of the donor to avoid the graft surgical disease.
First, we implemented the genome editing strategies to deplete, at the same time, these two molecules, designing sgRNA started at the b2M and the TRAC constant region of the TCR. And as you can see in the left, we optimize this, reaching almost half of the cells double negative for those markers.
Moreover, we implemented the selection of these double knockout cells using the AutoMACS system. And after the purifications, we were able to have more than 95% double negative cells with really good recuperation yield, proving that this system could be combined with the generation of the CAR to generate these allogeneic CAR T cells.
So then we move and combine this CRISPR knockout of HLA and TCR together with the already optimized protocol that Begoña mentioned previously for the delivery of the CAR. We just adapted, by a single nucleofection of the activated T cells, combining the minicircle, the mRNA for the transposon system, together with the Cas9 and the guide mRNA as an RNP complex for the depletion of those two genes. And as you can see in the bottom left before the selections, we have almost 50% of CAR+ cells and 50% of them are double narrative. And after the selection, we are able to really enrich in the double negative cells, maintaining the expression of the CAR. Then we corroborated the activity of these CARS in comparison with wild-type CAR T cells. And you can see in the bottom right that they are equally effective both in vitro, inside the TCRKO cells, and in cells expressing CD33 and also in animal models of AML that we were able to increase the survival of the treated animals similarly to wild-type CAR T cells.
Then focusing on the safety of this approach, on top of the already mentioned better integration profile of the transpo cells, we also corroborated that the genome editing and the depletion of these two genes had no effect or deleterious effect because of the knocking down. As you can see, first we measure the double negative cells depleted from the HLA were able to be not recognized by allogeneic PBMCs, indicating that the host won’t recognize these cells and we wouldn't have immune rejection. And also those cells ridded of the TCR were not able to react against allogeneic PBMCs. and diseases indicated that we will be avoiding the graft surgical disease.
Moreover, on the right you can see the genomic analysis of target efficacy of our guide RNAs. As you can see, the guide RNAs that were used for the depletion of the HLA and the TCR were very specific. We know of targets on the T cells indicating that those cells will be genomically safe.
Finally, with this product, we are also now implementing the production GMP level with the aim to perform a clinical trial in AML patients or NDH patients, using these allogeneic CAR T cells as a bridge therapy for allogeneic extension transplantation. This is what we have planned. Here you have the scale, the classical 3+3 trial design that we will be happy to start as soon as possible. The use of CRISPR, the regular CRISPR systems, and by regular, I mean those that induce double-strand breaks, they are very efficient for knockout genes, but also they have some issues associated with the possible toxicity in the genome due to translocations. Since we are cutting in two different genes at two different locations in the genome, one possibility is that we have rearrangement of the genome, so that way we would have safety issues with this product.
This is why we also try to implement in the lab additional CRISPR systems that are not based on the double-strand cleavage. And in this sense, we optimize the count of the TCR and HLA by using base editing approaches. Base editors are able to just modify single nucleotides. The cytosine base editors change Cs by Ts. The adenine base editors change As by Gs. In the case of the cytosine base editors, it's very easy to introduce stop codons without double-strand breaks into the genomes. Since some of the amino acids that code by CAA, for example, can be easily changed to TAA, we can induce stop codons or achieve the knockout of the expression of the gene, but without those double-strand breaks that will produce the translocations.
Here I summarize the implementation of these base editors instead of the regular CRISPR system for the generation of these allogeneic CAR T cells, and we have such good efficiencies generating double knockout cells that we are also able to reach and purify with almost the same efficacy in vitro and in vivo than the wild -type CAR T cells under regular CRISPR edited CAR T cells, indicating that this technology can also be implemented for the generation of allogeneic CAR T cells.
To sum up the second part: I hope I’ve convinced you that the CRISPR technologies provide a useful tool for the generation of double knockout cells for allogeneic approaches that can be combined with other state-of-the-art technologies like the transposon, even novel CAR designs, allowing the production of safe and efficient genome-edited CARs that will also reduce the manufacturing cost because we can use them in the same production for forever alone. And finally that the universal CAR T cells generated by the combination of CRISPR and transposon-based technologies can be produced at the GMP level, allowing the evaluation in clinical trials that we hope to start very soon.
I would like to thank all the people that have been involved in this project, the people from the Cima and CUN here at the University of Navarra. Also all the people from the CIEMAT, especially Begoña and Rosa, they were optimizing the protocol. And also the collaborators that we have, the clinical collaborators at IBSAL, the promoter of the clinical trial using the transposon-generated CAR T cells. Also the group of trial participants, they allow us to analyze the safety of the integration of the transposon vectors. Also MaxCyte for this opportunity to present this work and also for facilitating access to the machine and also the protocols and the optimization of the protocol. And of course, the funding agencies that allow us to perform our work. Finally, all of you for your attention.
So now we are open for any question that you have.
Jokūbas Leikauskas (moderator): Brilliant. Brilliant. That was really interesting. Thank you Begoña and Juan for such a great presentation. And now we're going to start our Q&A. So I would invite you to join me on camera and hope you're ready to answer some questions. So the first one is for you, Juan. Can the depletion of MHC I increase the elimination of CAR T cells by the activation of natural killer cell mediated cytotoxicity?
Juan Roberto Rodriguez-Madoz: Hello. Thank you for the question. This is one of the problems that the allogeneic CAR T cells has right now: you deplete the class one. So this is why we implemented this double knockout, but we really believe that using a combination of fully TCR, no culture combined with not fully depleted of class one could be a better approach in order to avoid this NK-mediated depletion of the HLA knockout cells.
But it's true, that is something that we have to have in mind, since it has already been described that NK could probably mediate this short-term persistence by the elimination of the edited cells.
Jokūbas Leikauskas (moderator): Great answer. Thanks Juan. That was really insightful, and now we have another one for you. So our audience member wants to know something related with the reduced persistence of the edited CAR T cells in vivo that has already been described.
Juan Roberto Rodriguez-Madoz: Up until now most of the clinical trials using allogeneic approaches, they have described that they have shorter persistence of the CAR T cell in the body. And this is something that we have to work more deeply on in order to improve the persistence of the CAR T cells. Since we have to knock out some of the genes that are involved in these toxicities for the allogeneic approaches, we should try to improve, I don't know if metabolically or just improving the metabolic state of the cells in order to increase the persistence and to have a really good expansion of the cells. The thing is that in the approach that we are using right now, since we are using the CAR T cells as a bridge therapy for a stem cell autologous transplantation, persistence is not a key issue in this particular setting. But if we want to use the allogeneic approaches for other diseases, this is something that will have to work on a little bit more.
Jokūbas Leikauskas (moderator): Amazing. That was a great answer. Thanks Juan, and now Begonia, we would like you to join the discussion as well. So one of our audience members wants to know, can you provide cost values for the GMP vectors?
Begoña Diez Cabezas: Thanks a lot for the question. If you want to go for a GMP-grade viral vector, it costs around 1 million euros for one small dose. So with our approach, we are trying to reduce this cost because the production of the viral vectors are one of the most expensive reagents that we use for the production of this kind of ATMP.
Jokūbas Leikauskas (moderator): Thank you, Begoña. And now we have more of a technical question next. So our audience member says, I noticed VCN of 10.6 in one of the engineering batch. How do you proceed or justify high VCN? US FDA wants VCN of less than five.
Begoña Diez Cabezas: I can answer this one. I have to say that we have been arguing a lot about this topic, especially because I have to say also that using transposons, it’s harder to control the vector copy number that you obtain in the cells. So the thing is, the vector copy number is quite high, but we just argue with our regulatory agencies that these cells are differentiated cells, so in the end, we don't really need a huge amount. We don't really control this copy number as much as with another kind of cell therapy product in which we modify cells, like stem cells that are going to be in the body of the patient for the rest of their lives. So we just accept that this number of copies is okay, and they agree with us and this is how we proceed.
Jokūbas Leikauskas (moderator): Thanks for your clarification, Begoña. That's good to know. And we have another question. So do you order all the preparation of the elements to be electroporated from the company, and how much does it cost?
Begoña Diez Cabezas: So at the beginning, we have done it in the lab, especially the in vitro mRNA for the transposase, but for the clinical trial, of course, we just order from a company that has a better standard of production for this kind of product. I don't remember [the exact cost] really well, but I have to say that this is really, really less money that you will spend on a GMP viral vector.
Jokūbas Leikauskas (moderator): Thanks a lot, Begoña. We actually have time for one more question. This one's for you, Juan. So our member wants to know, did you check for the frequency of chromosomal translocation that might be generated by double knockout?
Juan Roberto Rodriguez-Madoz: That is a really good question because it is something that is still pending. We have preliminary data just by PCR trying to check if we have the possible translocation within the onsite CLE sites. By qPCR, we haven't detected that, but it is true that we probably need to perform additional experiments with additional technological platforms or CAE or some of the new technologies that allow us to detect more deeply the possible translocations that could occur. But of course, it's something that has to be done, at least to verify that the protocol for the genome addition is safe, and we have a safe product for the clinical trial.
Jokūbas Leikauskas (moderator): Fantastic. Thank you both, everyone. That's all we've got time for today, unfortunately, but any questions we didn't get to, we will simply reply by email. And the webinar will be available on demand tomorrow, so look out for an email from us with a link. Also, we would like to invite you to take a short survey to provide us with feedback on today's webinar, the link to which can be found in resources on the righthand side of the screen or in the on-demand email as well.
And additionally, an attendance certificate is available on the righthand side too. So all that's left is to thank Begoña and Juan once more for such a great and insightful presentation. And thank you to our audience for listening. We hope you join us again very soon.