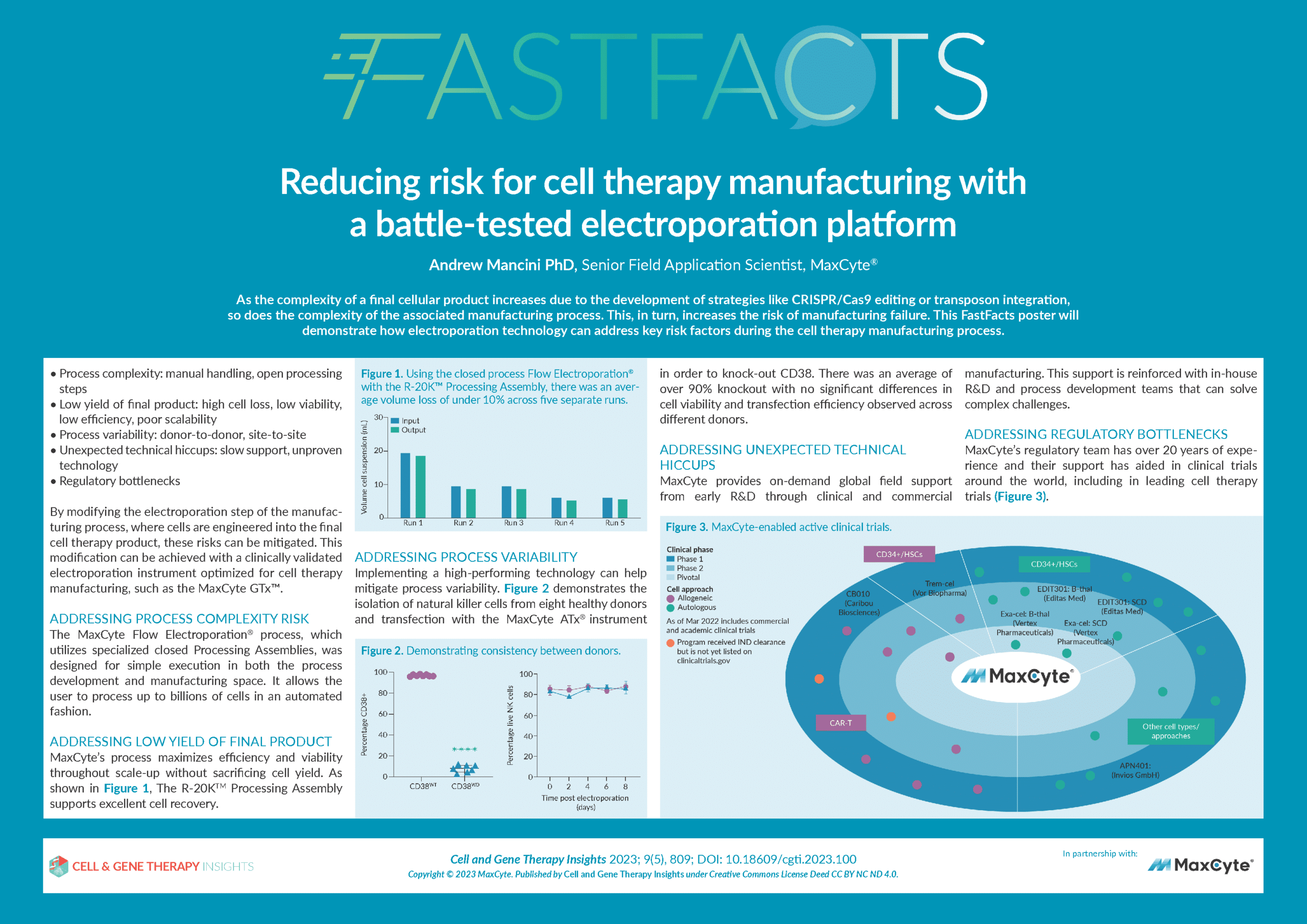
Reducing risk for cell therapy manufacturing with a battle-tested electroporation platform
Presentation
Watch this video to learn about:
- How choosing a clinically-validated manufacturing platform for critical steps such as cell electroporation can reduce risk during cell therapy manufacturing.
- The MaxCyte GTx™–a clinically-validated electroporation platform optimized for cell therapy manufacturing.
- How to address key risk factors during the cell therapy manufacturing process, such as process variability, complexity, and product yield.
- The importance of robust, expert technical and regulatory support in enhancing risk mitigation.
This video was broadcasted on Cell and Gene Therapy Insights on August 30, 2023.
Watch the presentation
Presenter






