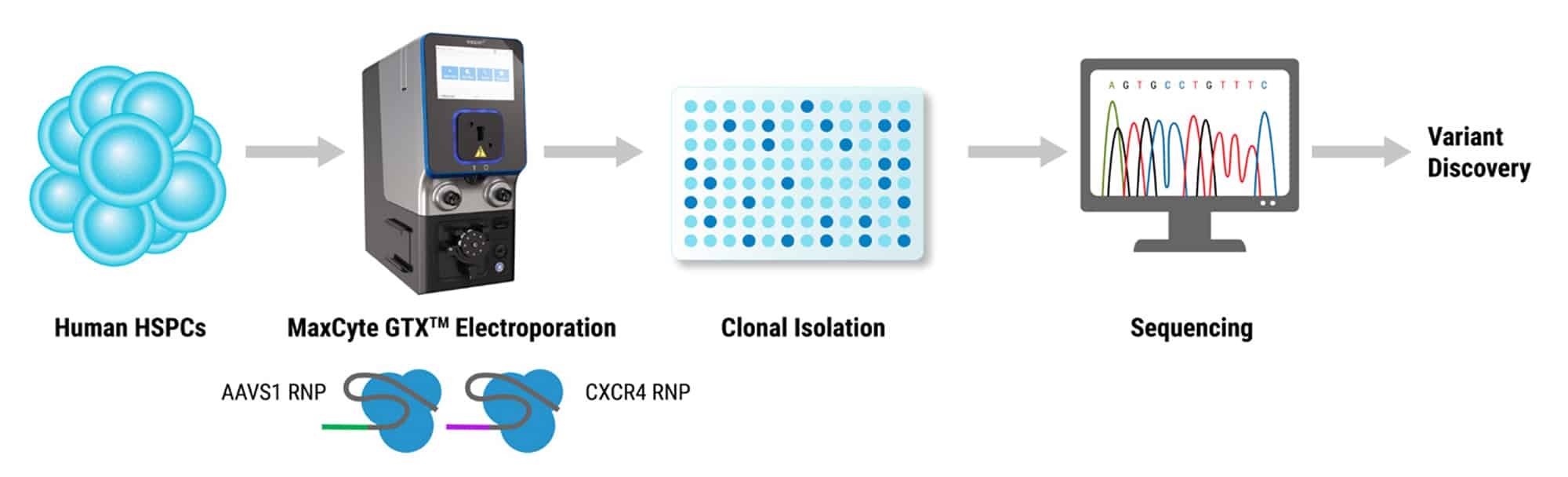
Reducing Risk in Gene Editing Programs through Robust Off-Target Safety Evaluation
Join us live for a webinar on how to assess off-targets for CRISPR-based gene editing applications
Tuesday, June 24, 2025
09:00 PDT; 12:00 EDT; 17:00 BST; 18:00 CEST
If you're engineering a CRISPR-based editing system for application in cell or gene therapy, this session is a must attend. You’ll gain insight into assembling an off-target safety package for your IND submission. For those in earlier stages of development, we’ll highlight how early assessment of off-target risks can help de-risk your program.
The session will feature presentations from Dr. Stephanie Cherqui (Papillon Therapeutics) and Dr. Colin Exline (Papillon Therapeutics), who will share their experience advancing a Friedreich’s Ataxia program toward IND submission, including a preview of their off-target safety data package. Dr. Vijetha Vemulapalli (MaxCyte - SeQure DX) will provide a comprehensive overview of the current tools used to generate robust off-target data for CRISPR/Cas and base editing systems.
Whether you’re in early discovery or preparing for IND-enabling studies, this session offers practical guidance rooted in real-world experience. Join us to gain strategic insights, learn from experts, and strengthen the safety foundation of your CRISPR-based therapeutic program.
Presenters



Have more questions?
Send your question to one of our cell engineering experts.