A cGMP Compatible, Non-Viral CAR T Cell Manufacturing Process
Webinar
Abstract
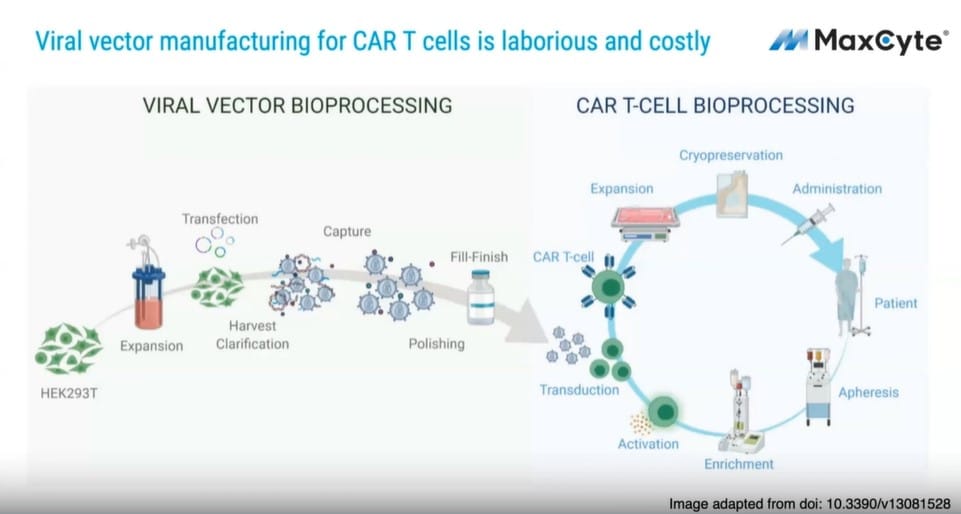
Recent breakthroughs in autologous cellular therapies have generated tremendous excitement for the industry; however, these advances have been confronted with the expense of manufacturing of viral gene delivery and concerns over random integration and the safety of viral vectors. To combat these challenges, researchers at the University of California San Francisco have developed a novel system that offers lower toxicity with a single-stranded DNA (ssDNA) approach.
UCSF scientists used MaxCyte ExPERT technology as a non-viral alternative that allows for site-specific delivery of transgenes through homology-directed repair (HDR) to circumvent complex and expensive manufacturing. This approach resulted in highly efficient knock-in integration efficiencies (46-62%) and yields of 1.5 x 109 CAR+ T cells, well above current adoptive cellular therapy doses.
The groundbreaking experiments discussed during this event demonstrate the ability of the MaxCyte GTx™ to deliver ssCTS HDR templates and CRISPR-Cas9 RNP to modify T cells in clinically relevant amounts.
Learning objectives
- Outline large-scale, non-viral manufacturing for engineered CAR T cells
- Demonstrate 5-fold boosted knock-in and >7-fold increased live cell yields relative to dsDNA approaches
- Reveal MaxCyte GTx™ delivery of ssCTS HDR templates and CRISPR-Cas9 RNP to modify T cells for a future clinical setting
Watch Webinar
Presenter







