Efficient Scalable Manufacturing of Virus-like Particles for the Delivery of CRISPR-Cas9 Ribonucleoproteins using a cGMP-Compliant Electroporation Platform
American Society of Gene and Cell Therapy Annual Meeting (ASGCT) Poster Presentation
Baltimore, Maryland, USA
May 10, 2024
Our MaxCyte scientist, Isabel Daher, presents her poster from the 2024 American Society of Gene and Cell Therapy annual meeting
Genome editing tools such as CRISPR-Cas9 nucleases, base editors, and prime editors hold tremendous promise for treating human diseases by being able to specifically modify a targeted region of the DNA genome. However, there are limited in vivo delivery options that are safe, efficient and transient. Moreover, viral vectors such as AAV are hampered by limited cargo size capacity. Virus-like particles (VLPs) offer a solution to these problems. VLPs are derived from retroviral structural proteins, which can be engineered to specifically package a cargo of interest. They are safer than traditional viral vectors because they lack a viral genome but can utilize the traditional virus delivery machinery to target and enter cells. Recently, VLPs have been reported to efficiently package base editors and prime editors for delivery into mice at therapeutically relevant levels. To realize the potential of VLPs for in vivo delivery, alternative methods to scale up their manufacturing will be critical for future clinical applications. Here, we utilized the MaxCyte ExPERT GTx, a cGMP-compliant electroporation instrument, to manufacture VLPs—packaged with CRISPR-Cas9 RNPs for genome editing in target cells—in adherent and suspension HEK293 cells. We found that electroporation consistently produced significantly higher yields of functional VLPs compared to a commercially available transfection reagent, with up to a 20-fold improvement when using an optimized electroporation protocol. Furthermore, the production of VLPs using electroporation exhibited favorable production kinetics compared to other transfection methods, enabling a shorter VLP manufacturing process. Finally, we demonstrated the scalability of VLP production across a 15-fold volume range with minimal re-optimization. In summary, our results show that electroporation is a viable means for consistent, efficient and scalable manufacturing of VLPs for gene-editing applications and has high promise to address needs for future clinical and commercial VLP manufacturing.
Key takeaways
- Transfect up to 100 billion cells using a cGMP-compliant method that can enable rapid clinical manufacturing
- Produce functional VLPs in both adherent and suspension HEK293 cells
- Multiple performance parameters were superior using electroporation over chemical transfection
Watch Isabel's poster presentation
View the complete poster that established an optimized process for manufacturing virus-like particles using the MaxCyte's Flow Electroporation technology.
Have more questions?
Send your question to one of our cell engineering experts.
Presenter

Related resources
Poster presentation transcript
Hello, and welcome to MaxCyte. My name is Isabel Daher, and I'm a research associate in the Technical Applications Lab. Welcome to our presentation, where we would like to share with you a new and efficient, scalable manufacturing process to produce virus-like particles for the delivery of CRISPR-Cas9 ribonucleoproteins using our cGMP-compliant electroporation platform.
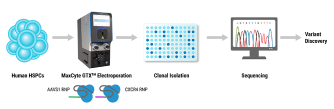
Our production workflow first starts with combining our producer cells, which are either adherent or suspension HEK293 cells with a four-plasmid mix. Here, we use the murine leukemia virus capsid and are targeting the HEK3 loci for proof of concept. The cells and cargo are then loaded into a processing assembly, which is then inserted into the instrument for electroporation with our preloaded and optimized protocol.
The cells are then allowed to rest and recover at high density before culture media is added. The VLPs are then harvested from the culture supernatant one or two days post transfection, followed by concentration, purification, and cryopreservation. To assess our VLP yields, the VLPs are titrated onto adherent HEK293 cells, genomic DNA is isolated three days post transduction, and the percentage of insertions and deletions, or indels, are quantified via Sanger sequencing and analysis with the Synthego ICE tool.
In electroporation, there are two main parameters that need to be optimized. The first is DNA concentration, and the second is electroporation energy. In a dose-dependent manner, increasing total DNA concentration improves VLP yield up to a point where the DNA becomes too toxic and cell viability decreases. Next, looking at electroporation energy, we show that increasing total energy is correlated with higher VLP yields and thus higher editing efficiency.
We also wanted to explore if the addition of a chemical enhancer to the production media would enhance VLP production, and in fact, almost double the VLP yield, reported here as editing activity.
Now that we have an optimized electroporation protocol, we are ready to benchmark it against a commercially available transfection reagent. Looking at day two post transfection without the presence of any chemical enhancer, we see that, while chemical transfection can produce VLPs that induce approximately 60% indels, electroporation was able to produce VLPs that induce up to 95% indels, and at a much lower dose!
This is also reflected in our ELISA measurements where we titer the VLPs based on the capsid core antigen p30. Now, to compare the kinetics of production between chemical transfection and electroporation, we see that VLPs produced with chemical transfection exhibit a delayed kinetic profile as compared to electroporation, where editing activity reaches upwards of 95% even by one day post electroporation.
In addition to producing VLPs on a shorter timeline, electroporation enables improved cargo packaging, as shown in the immunoblot where sample inputs were normalized to p30, measured via ELISA, and then probed with an anti-Cas9 antibody. This data highlights the key difference in transfection efficiency between methods and further supports our electroporation platform for VLP production.
Now that we've established a reproducible workflow in adherent HEK cells, we wanted to transition to suspension HEK293 cells as that is what typically is used in clinical and commercial manufacturing.
We tested the same protocols previously designed for adherent HEK cells and found that they are transferable with no need to re-optimize. We also found that in suspension cells, VLP production is actually more favorable when harvesting on day one post transfection as compared to any later time point as we hypothesize that the nascent VLPs are transducing the producer cells as they expand in culture.
Here, we show that the same chemical enhancer used in adherent HEKs can be used in suspension cells to improve VLP production, and that the producer cells can be cultured at high densities up to a three-fold increase from the default, to minimize production culture volume in a bioreactor.
Again, we compare our production workflow in suspension HEK cells to a commercially available transfection reagent. We find that even in the absence of a chemical enhancer, electroporation outperforms chemical-transfection-mediated VLP production, which, at best, could approach only 40% indel formation on day one post transfection.
Now, to look at the scalability of our production workflow, we compared different scales of electroporation with matched conditions for chemical transfection. We demonstrate that electroporation is seamlessly scalable and produces VLPs with similar editing efficiencies, irrespective of the scale of production. And again, electroporation is superior to chemical transfection when producing competent VLPs in suspension HEK cells. The editing efficiencies of MaxCyte-enabled VLPs reach upwards of 95% on day one, even without the need for a chemical enhancer. And, this is reflected by the capsid titers, where VLPs produced with electroporation exhibit over ten-fold improvement in titer compared to chemical transfection on day one and a seven-fold improvement on day two.
Taken together, we demonstrate that MaxCyte's cGMP-compliant electroporation platform can transfect up to 100 billion cells, which would enable rapid clinical manufacturing. We also show that our platform is efficient and scalable for competent VLP production and can be used for both adherent and suspension HEK293 cells. Finally, MaxCyte's electroporation workflow is a simple time-saving alternative to chemical transfection for VLP production.
We thank you for your time, and we hope to enable your VLP production workflows soon. Please reach out with any questions. Thank you.