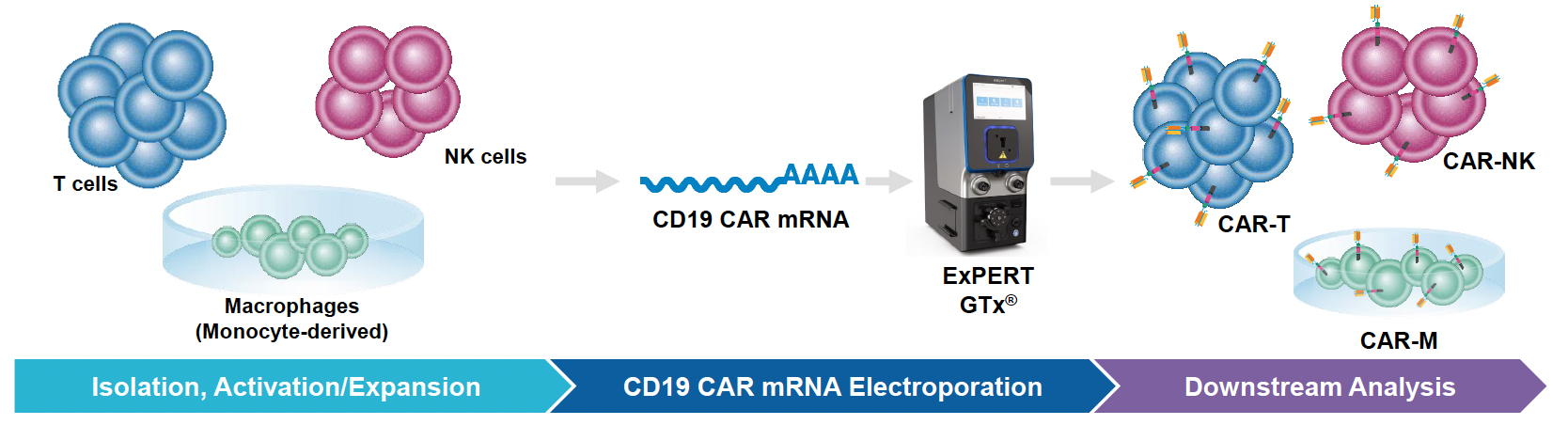
MaxCyte® Electroporation Enables CRISPR/Cas-Mediated CAR Knockin in Primary Human T Cells
American Society of Gene and Cell Therapy Annual Meeting
New Orleans, Louisiana, USA
May 15, 2025
MaxCyte scientist Ashley Strickland-Dietz presents protocols to improve non-viral engineering of CAR T cells
Abstract
In recent years, chimeric antigen receptor (CAR) T cells have emerged as a leading treatment for various hematological cancers. Despite demonstrating great promise, there are several challenges that limit CAR T cell therapies, including concerns regarding efficacy, safety and manufacturability.
To address these, groups have turned to non-viral engineering of CAR T cells using CRISPR gene editing. In addition to reduced immunogenicity and manufacturing costs, this technology enables precise knockin of tumor-targeting receptors and knockout of genes responsible for rejection, toxicity and immunosuppression. To CRISPR engineer CAR T cells, Cas nucleases, guide RNA (gRNA) and homology-directed repair (HDR) template, DNA must be efficiently delivered into T cells without jeopardizing their viability or functionality.
With this in mind, we sought to generate optimized workflows that enable CRISPR-mediated gene editing in primary human T cells using MaxCyte electroporation.
To this end, we first identified optimal electroporation protocols and concentrations of sgRNA, Cas9 and HDR template required to knock in GFP at the TRAC locus. Next, we compared different repair template designs, including the addition of Cas9 targeting sequences (CTS). We also investigated multiple HDR template DNA formats, including conventional plasmids and Nanoplasmids™. In addition, we explored the use of small molecule enhancers and identified several that further improved editing efficiencies.
These findings were then used to engineer CD19-targeting CAR T cells. With activated T cells from healthy donors, we could achieve CAR expression levels of greater than 70%. In addition to efficient and reproducible CAR knockin in T cells from multiple donors, cells engineered using this workflow were viable and retained the ability to expand and eradicate CD19-expressing target cells.
Together, these demonstrate the capability of MaxCyte’s clinically validated ExPERT electroporation technology to enable non-viral engineering of CAR T cells using the CRISPR/Cas system.
Key takeaways
- MaxCyte electroporation enables stable expression of tumor-targeting receptors in human T cells while maintaining high cell viabilities and functionality.
- Smaller HDRTs, the addition of a CTS and the use of HDR enhancers can be used alone or in combination to improve gene knockin.
- The ExPERT GTx® reproducibly engineers CAR T cells without impacting expansion, and these cells are functional and highly effective at eradicating tumor cells.
- MaxCyte's optimized electroporation protocols can be used to enhance gene knockin without the need for HDR enhancers.
Watch the presentation on optimizing CRISPR/Cas-mediated CAR knockin in T cells

View more details and illustrations explaining how MaxCyte's electroporation platform optimizes CRISPR-based CAR knockin for primary human T cells
Have more questions?
Send your question to one of our cell engineering experts.
Presenter

Poster presentation transcript
Hello, my name is Ashley Strickland-Dietz. I'm the immunology scientist at MaxCyte and this year at the American Society of Gene and Cell Therapy Conference in New Orleans, I presented our work on optimizing CRISPR/Cas-mediated CAR knockin in primary human T cells using MaxCyte electroporation.
In recent years, chimeric antigen receptor T cells have emerged as a leading treatment for various hematological cancers. Despite demonstrating great promise, there are several challenges that limit CAR T cell therapies, including concerns regarding efficacy, safety and manufacturability. To address these, groups have turned to non-viral engineering of CAR T cells using CRISPR gene editing. In addition to reduced immunogenicity and manufacturing costs, this technology enables precise knockin of tumor-targeting receptors and knockout of genes responsible for rejection, toxicity and immunosuppression.
To CRISPR engineer CAR T cells, Cas nucleases, guide RNA and homology-directed repair template, DNA must be efficiently delivered into T cells without jeopardizing their viability or functionality. With this in mind, we sought to generate optimized workflows that enable CRISPR-mediated gene editing and primary human T cells using MaxCyte electroporation.
To this end, we first identified optimal electroporation protocols required to deliver DNA into activated T cells. For this, human T cells were isolated and activated for 48 hours then transfected with 50, 100 or 200 micrograms per mil of a plasmid encoding GFP using one of two electroporation protocols commonly used for DNA delivery: expanded T cell three, abbreviated ETC3, and expanded T cell four, abbreviated ETC4.
We observed that transfection efficiencies and GFP expression were increased as DNA concentration increased. As expected, DNA transfection was accompanied by a drop in viability, but cells quickly recovered by 48 hours post electroporation. Overall, at all DNA concentrations tested, ETC4 gave superior loading efficiencies without a dramatic decrease in viability compared to ETC3. For these reasons, we used ETC4 for all subsequent experiments unless otherwise specified.
Next, we identified concentrations of Cas9 and guide RNA required for optimal TRAC knockout. For this, activated T cells were transfected with varying molar ratios of Cas9 to guide RNA, including one to 0.5, one to one, one to two, and one to three, each at differing final RNP concentrations including 0.5, one, two and four micromolar.
At day two post electroporation, there were slight differences between the conditions tested, though all samples achieved greater than 80% TRAC knockout. And by day four, knockout efficiencies were greater than 95% for all conditions. To reduce the volume of RNP needed, we selected 0.5 micromolar as our final RNP concentration, and because it gave a slight improvement in TRAC knockout at day two, we chose to use a Cas9 to guide RNA ratio of one to two.
Having identified the conditions needed for TRAC knockout, we next wanted to determine a suitable HDR template concentration to achieve good levels of gene knockin. For this, we co-transfected activated T cells with the TRAC-targeting RNP, using the conditions previously identified, along with 50, 100 or 200 micrograms per mil of a plasmid HDR template for GFP knockin.
At both days four and seven post electroporation, increasing concentrations of HDR template resulted in increasing knockin, with 200 micrograms per mil, giving the best efficiency and expression without a significantly higher impact on viability. Based on these experiments, we established the following workflow for CRISPR knockin in activated T cells using MaxCyte’s ExPERT GTx.
Briefly, T cells are isolated and activated for 48 hours. Activated T cells are then harvested, resuspended in MaxCyte electroporation buffer and co-transfected with 0.5 micromolar RNP at a one to two Cas9 to guide RNA ratio along with 200 micrograms per mil of HDR template using the electroporation protocol ETC4. Transfected cells are then expanded in culture and assessed via downstream assays.
Having established this workflow, we next sought to improve knockin. For this, we first tested different HDR template formats. In particular, we tested a traditional plasmid with and without a Cas9 targeting sequence, or CTS, as well as a Nanoplasmid HDR template with and without a CTS, all for GFP knockin.
At both day two and day five post EP, the smaller Nanoplasmid template gave improved knockin over traditional plasmids, regardless of the presence of a CTS. The addition of a CTS also improved knockin for both the larger plasmid and smaller Nanoplasmid templates, demonstrating two HDR template modifications that can be used to improve knockin.
Next, we examined whether small molecule HDR enhancers could be used to improve knockin. For this M3814 and AZD7648 were added post electroporation at different concentrations to activated T cells. Co-transfected with TRAC RNP and a plasmid HDR template for GFP knockin. At day four post electroporation, both enhancers improve knockin in a dose dependent manner, though this did come at a slight cost of viability. Two micromolar of M3814 improved knockin the most with little to no additional hit to viability and was selected as the enhancer condition for all subsequent experiments unless otherwise specified.
We then combined these strategies—smaller HDR templates, the addition of a CTS sequence and the use of HDR enhancers—and found that these could be used in combination to further improve gene knockin with the smaller Nanoplasmid template plus a CTS and two micromolar of M3814, achieving the highest levels of GFP knockin at both day four and day seven post ep.
This workflow was then used to generate CD19 CAR T cells. For this, activated T cells from three different healthy donors were co-transfected with TRAC RNP and either a plasmid or Nanoplasmid™ template for CAR knockin. While there was donor to donor variation, MaxCyte did enable TRAC knockout and CAR knockin in all three donors, demonstrating the robustness of this workflow and the ability for MaxCyte to engineer therapeutically relevant CAR T cells.
In the donor achieving the highest knockin, efficiencies approached 70% by day seven using a Nanoplasmid template and enhancer. Using the same donor, we once again engineered CAR T cells, this time using four different HDR template formats, including plasmids and Nanoplasmids with and without a CTS, and expanded the cells for 14 days post electroporation. As before, we achieved knockin efficiencies between 35 and 86% between days four and 14 with sustained CD19 CAR expression, demonstrating the reproducibility of this workflow.
While variabilities were initially impacted by the editing event, all recovered quickly and remained high. More importantly, MaxCyte did not impair the ability for total T cells or CAR-positive T cells to expand.
For this, activated T cells from a different donor were co-transfected with TRAC RNP and Nanoplasmid HDR template for CAR knockin. Once again, MaxCyte enabled knockin efficiencies between 52 and 69% without enhancer and between 67 and 89% with enhancer. On day 14, these cells were co-incubated with CD19-expressing Ramos cells at varying effector to target ratios, including 0.25 to one, 0.5 to one, one to one, and two to one. At 24 hours, target cell killing and granzyme B and interferon gamma [IFN-γ] expression were assessed. CAR T cells engineered using MaxCyte electroporation were functional, eradicating target cells and expressing both granzyme B and IFN-γ.
While we were able to achieve good gene knockin, especially with M3814, some groups prefer to avoid the use of small molecules. For these reasons, we sought to further optimize our electroporation protocols to enable high levels of knockin without the need for an HDR enhancer. For this, activated T cells were co-transfected with TRAC RNP and plasmid HDR template for GFP knockin. At day four post EP, compared to expanded T cell, for modified protocols one through four, all enabled greater levels of knockin without the use of enhancers.
Though this improvement sometimes came at the cost of viability, efforts are ongoing to develop an optimized electroporation protocol for CRISPR knockin in activated T cells with high levels of gene editing and minimal impact on viability.
In summary, MaxCyte Electroporation enables stable expression of tumor-targeting receptors in human T cells while maintaining high cell viabilities and functionality. Smaller HDR templates as well as the addition of a CTS and the use of HDR enhancers can be used alone or in combination to improve gene knockin. The ExPERT GTx reproducibly engineers CAR T cells without impacting expansion, and these cells are functional and highly effective at eradicating tumor cells. MaxCyte’s optimized electroporation protocols can be used to enhance gene knockin without the need for HDR enhancers.
And with that, I'd like to thank you for listening to this presentation, and if you have any questions regarding MaxCyte’s capabilities to enable CRISPR gene editing, please feel free to reach out.