Assay Ready Cells for Cell-Based Assays
Cell-based assays can streamline research and drug discovery by enabling scientists to interrogate a target in a physiological context. Developing stable cell lines for use in cell-based assays is expensive, time-consuming, labor-intensive and imposes constraints on the cell types that can be used.
With MaxCyte® electroporation technology, transiently transfected Assay Ready Cells can be a time-saving, cost-effective tool to develop new cell-based assays, to rapidly adapt existing assays to new targets and to run screening programs.
Accelerate Drug Discovery with Assay Ready Electroporated Cells
Drug discovery remains a challenging process that can take over a decade from concept to commercialization, with costs often exceeding $1 billion, and it comes with no guarantee of success.1 Recent years have seen an increase in the use of cell-based assays during the hit and lead discovery phases as an alternative or complementary method to biochemical or 'isolated target' assays.2
Cell-based assays can streamline research and drug discovery by enabling scientists to interrogate a target in a physiological context. This ability offers clear benefits in many cases, potentially leading to the identification of better-quality drug candidates. Cell-based assays may be the only viable screening solution for some complex targets that are challenging or impossible to purify.
Historically, cell-based assays have relied on engineered cell lines that stably express or over-express the target of interest and the required assay components. However, developing stable cell lines is expensive, time-consuming, and labor-intensive and imposes constraints on the target that can be studied and the cell types that can be used. Long-term culture of stable cell lines also comes with the risks of cell culture contamination and drift in expression levels.
Using transiently transfected cells reduces these risks and can facilitate the expression of challenging targets such as multi-subunit complexes and toxic proteins. However, batch-to-batch variability may be a concern if multiple transfections are needed to generate sufficient cells for large screens.
Assay Ready Cells can be a time-saving, cost-effective tool for assay development and screening. Using MaxCyte Flow Electroporation®, a single batch of billions of Assay Ready Cells can be prepared and then cryopreserved at the desired cell number and concentration. When needed, cells are revived and ready to use, typically within 24 hours.
MaxCyte electroporation technology provides reproducible, scalable transfection of virtually any cell type, enabling assay development and the production of Assay Ready Cells in pathologically relevant cell types, including primary cells, stem cells and cells of hematopoietic origin.
Although MaxCyte electroporation has minimal impact on cells, there are several other factors discussed here that should be optimized when preparing Assay Ready Cells for cell-based assay development.
1. Hughes, J., Rees, S., Kalindjian, S. and Philpott, K. (2011), Principles of early drug discovery. British Journal of Pharmacology, 162:1239-1249.
https://doi.org/10.1111/j.1476-5381.2010.01127.x
2. Wei F, Wang S, Gou X. A review for cell-based screening methods in drug discovery. Biophys Rep. 2021 Dec 31;7(6):504-516. doi: 10.52601/bpr.2021.210042. PMID: 37288368; PMCID: PMC10210057.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10210057/
Cell Culture and Handling
Low passage, healthy cells in log phase growth are essential to achieve the best results when preparing Assay Ready Cells; freshly thawed and late passage cells generally do not transfect efficiently.
Immediately after transfection, electroporated cells should be rested at 37°C for 20 minutes, preferably in an empty culture vessel, before the addition of media or cryopreservation.
MaxCyte processing assemblies (PAs) were designed to maximize cell recovery; there is no need to rinse out the PA to try to collect more cells.
DNA Quality
DNA quality affects both transfection efficiency and cell viability.
Plasmid DNA can be prepared using commercially available kits and should be resuspended in water at a high concentration, preferably greater than 5 mg/mL.
To produce electroporated cells, the DNA used should be endotoxin-free, and quality should be confirmed by gel electrophoresis and absorbance ratios.
DNA Concentration
Cell viability is inversely correlated with the quantity of DNA loaded into the cell due to DNA toxicity.
Users should perform a DNA titration experiment using small-scale electroporation for each new assay, cell type, target or loading agent to identify the DNA concentration that yields the optimal balance of assay responsiveness and cell viability.
Once optimized, the protocol to generate electroporated cells can be scaled seamlessly to transfect up to 2x1010 cells via Flow ElectroporationTM without needing re-optimization.
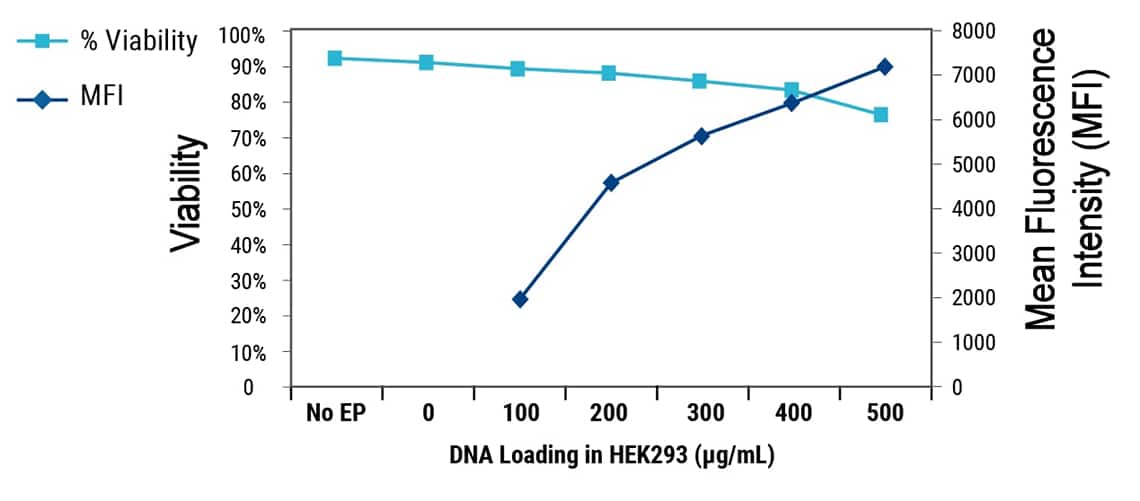
Inverse correlation between transgene expression and cell viability in transiently transfected HEK 293H cells.
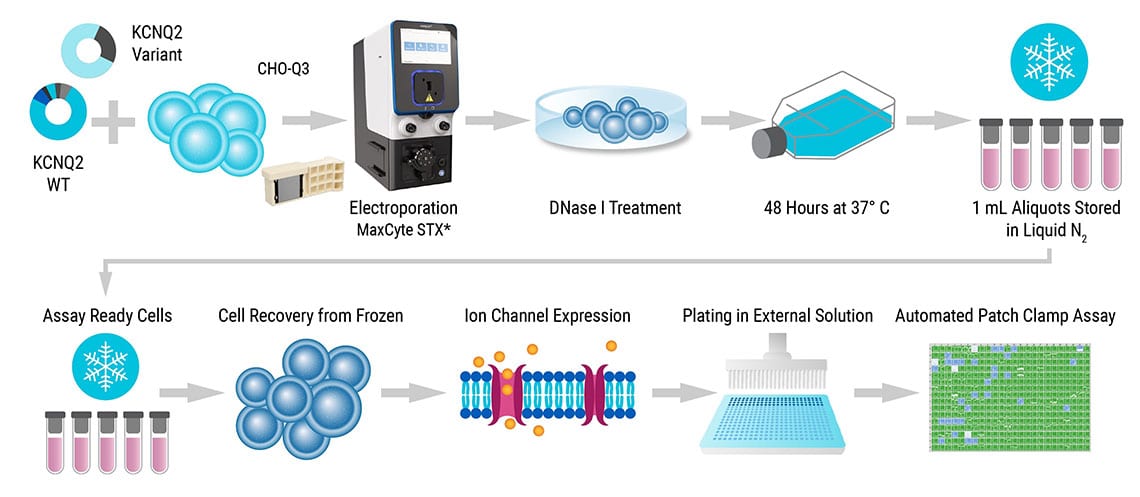
Learn how researchers prepared and used cryopreserved stocks of electroporated cells to enable the high-throughput evaluation of epilepsy-associated ion channel variants.
Adapted from Vanoye et al. JCI Insight. 2022 under Creative Commons License Attribution 4.0 International (CC BY 4.0)
Plating and Analysis
Cell plating conditions and the time between transfection and analysis can impact the responsiveness of cell-based assays. We recommend that these parameters should be optimized for each new assay.
Interestingly, some researchers report that lowering the culture temperature of electroporated cells to 28°C several hours after transfection leads to higher cell surface expression of ion channels, which may be beneficial in patch clamp assays.
Optimizing Cryopreservation of Electroporated Cells
MaxCyte electroporation can be used to generate Assay Ready Cell banks by transfecting cells in bulk, and then cryopreserving aliquots of the electroporated cells for future use. Different cell types, targets and assay applications require different cryopreservation regimens; users should test several conditions to determine what works best for each new combination.
Assay Ready Cells are typically frozen in 10% DMSO plus serum (20-90% v/v) or commercially available cryopreservation solutions for long-term storage in liquid nitrogen.
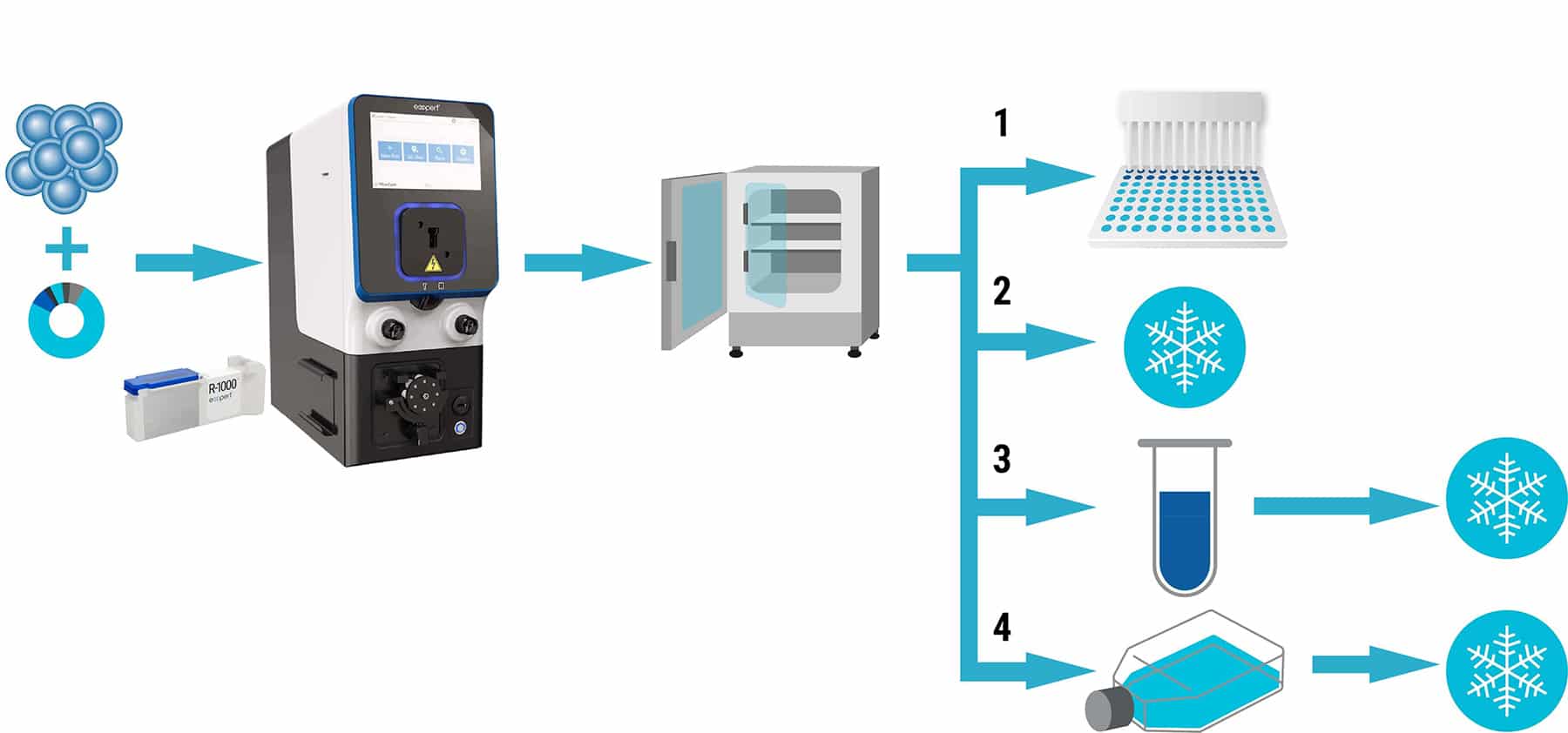
Workflow overview for optimizing cryopreservation conditions. After electroporation in an OC-400 or R-1000 processing assembly, cells are rested at 37°C for 20 minutes, then divided into four treatment groups: 1) plate without freezing and assay at 2-3 time points after transfection; 2) freeze immediately, recover frozen cells overnight then assay; 3) incubate in medium for 1-2 hours before freezing; and 4) plate 18-48 hours before freezing, recover frozen cells then assay immediately.
Assay Ready Cells for Any Cell-Based Assay
Electroporated cells can be used for virtually any cell-based assay application
- Nuclear Receptor Assays
- Kinase Assays
- Reporter Assays
- High Content Screening
Electroporation Systems
The MaxCyte ExPERT STx® and ATxTM are the next generation of the industry's leading scalable electroporation technology for seamless transition from R&D to screening. The ATx and STx enable small to large-scale transfection of virtually any cell type for the rapid development of cell-based assays without the need for stable cell lines.


Reagents and Processing Assemblies
MaxCyte's consumable products provide users with a variety of options for project scale and throughput from discovery through cGMP manufacturing using a single platform. Our range of Processing Assemblies allows users to transfect a variety of cell sample volumes to meet specific application needs. MaxCyte's Electroporation Buffer is animal-derived component free and safe for all cell types ensuring consistent, high-performance transfection.
Still have questions about cell-based assays and Assay Ready Cells?
Find out how we can help you reach your Drug Discovery goals.







