The 31st Annual Meeting of the Japan Society of Gene and Cell Therapy (JSGCT2025) will take place from July 23 to 25, 2025, at Hotel Gajoen Tokyo. With the theme “International Contributions and the Dawn of a New Era,” the meeting will gather experts from around the world to discuss the latest developments and challenges in gene and cell therapy. As the field rapidly advances—especially in cancer and genetic disorder treatments—issues such as domestic development delays and talent shortages are becoming more prominent. JSGCT2025 offers a vital forum for global collaboration, the promotion of Japanese technologies, and cross-sector dialogue among academia, industry and regulators.
Featured presentation
Afternoon Seminar #3: Advancing Cell and Gene Therapies Through Non-Viral Engineering Modalities
Wednesday, July 23, 2025, from 3:15 – 4:15 p.m. in Hall 3 (Hanashirabe)
Genome engineering of primary human cells holds great promise for the treatment of diseases including cancer or genetic disorders. However, viral cell engineering has several limitations, including high cost, lengthy manufacturing processes and the risk associated with viral integration into the host genome. Therefore, a non-viral genome editing approach is gaining traction from basic to clinical research. MaxCyte® electroporation efficiently delivers genome editing tools in the form of DNA, mRNA and RNP into a wide variety of primary cells and stem cells. In this session, we outline key considerations for delivering different types of cargo in primary cells and highlight examples of high editing efficiency in human keratinocytes, iPSCs, NK cells and activated T cells for knockout and knockin applications while maintaining high cell viability.
Presenters


Poster presentation
#LP5: Highly Efficient Engineering of Difficult-to-Transfect Immune Cells Using MaxCyte® Electroporation

Poster presented by Peter Gee, PhD, APAC Regional Manager of Field Applications at MaxCyte
Thursday, July 24, 2025, 10:55 – 11:35 a.m.
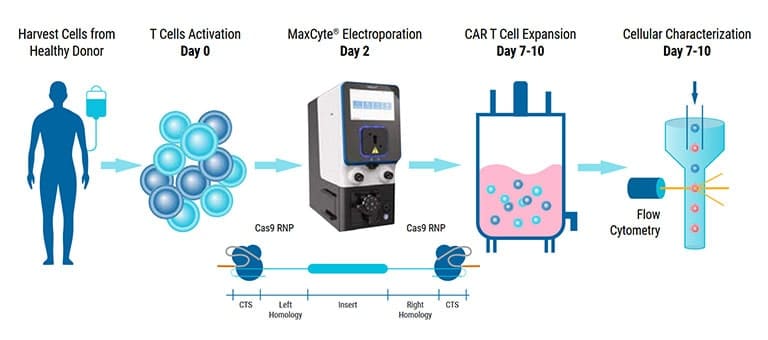
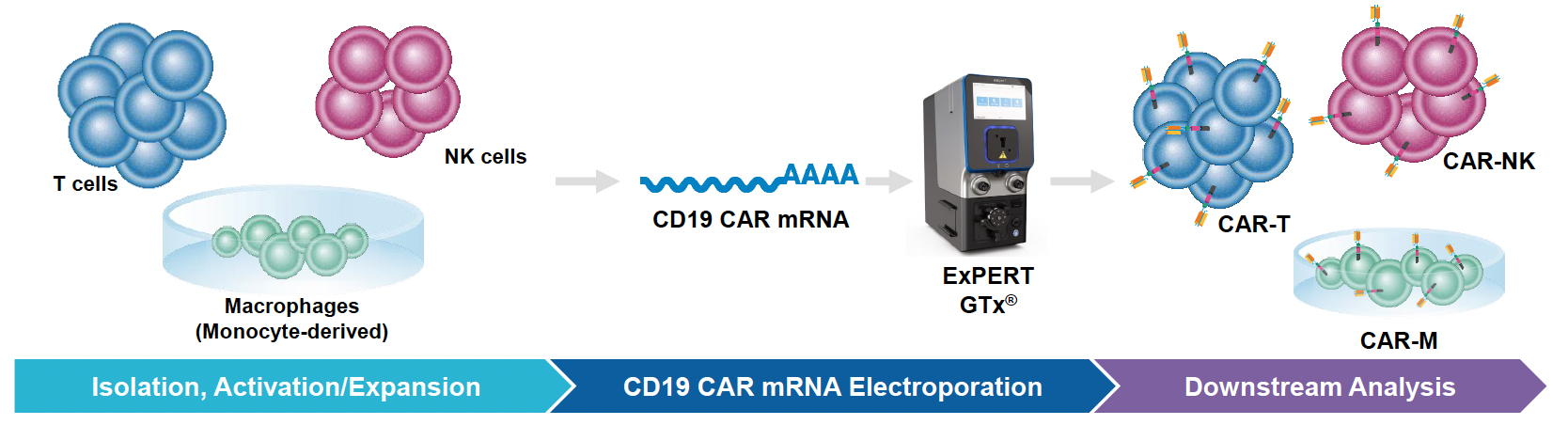
Since their inception, cell-based therapies have emerged as promising treatments for a wide range of diseases. Indeed, immune cells such as T cells, NK cells and macrophages are being used to treat various cancers, autoimmune disorders and degenerative diseases. To engineer these cells for improved efficacy and safety, biomolecules and other genome editing tools must be delivered into these difficult-to-transfect cells. To this end, MaxCyte has developed optimized cell engineering workflows using the ExPERT™ electroporation platform that enables highly efficient delivery of molecules, such as RNA, DNA and CRISPR-Cas nucleases, into a variety of different cell types. Here, we demonstrate that these workflows can be used to engineer primary human immune cells to express tumor-targeting receptors while maintaining high cell viabilities and functionality. In particular, MaxCyte enabled transient and stable expression of CARs/TCRs in T cells, NK cells and macrophages through high-efficiency transfection of mRNA, DNA encoding transposons/transposases, or CRISPR ribonucleoproteins (RNPs) and homology-directed repair (HDR) templates into these hard-to-transfect cells. In addition, these workflows seamlessly scaled up, allowing these cells to be engineered at therapeutically relevant scales.