From advances in cell therapy to changes at MaxCyte®, the past few months have been busy. Through it all, we remain committed to helping you solve the toughest cell engineering challenges with our proven ExPERT™ platform and trusted support. This issue of MaxCyte Minutes Newsletter has a lot in store! Let’s dive in.
In the News

MaxCyte® Acquires SeQure DXTM to Broaden Cell Engineering Offerings with On-target and Off-target Editing Assessments
MaxCyte Signs Strategic Platform License with TG Therapeutics to Advance its Autoimmune Cell Therapeutics Programs
TG Therapeutics will use MaxCyte’s Flow Electroporation® technology and ExPERT™ platform to support the development and commercialization of azer-cel, its allogeneic CD19 CAR T cell therapy program, for the treatment of autoimmune diseases.
TG received clearance by the U.S. Food and Drug Administration (FDA) of an Investigational New Drug (IND) application for azer-cel in progressive forms of multiple sclerosis (MS) and is targeting commencement of a Phase 1 trial in 2025.
Maher Masoud, MaxCyte President and CEO added “Our technology has been integral to the manufacturing of allogeneic T cell immunotherapies and was efficiently transferred from Precision BioSciences when TG Therapeutics obtained global rights for azer-cel for autoimmune diseases in January 2024. With our new partnership, we will continue to support the development of azer-cel to expand the application to autoimmune diseases.”
Your Success, Our Partnership
We’ve updated our website to put our partnership model front and center—because we believe it’s one of the most important reasons cell therapy developers choose MaxCyte. Our new pages break down how our Strategic Platform License (SPL) isn’t just a licensing agreement—it’s a proven accelerator, designed to help you move faster, reduce risk, and scale with confidence from early development through commercial launch. Explore what makes our partnership different.
Streamline Your Manufacturing with Seamless DCS Integration
- Streamline processes by integrating electroporation into your overarching DCS.
- Enhance data integrity with real-time acquisition and management.
- Ensure regulatory compliance with comprehensive audit trails and accuracy.
Exploring Non-Viral Gene Therapy Techniques
Read the latest eBook to learn how non-viral gene therapy techniques are revolutionizing drug development. These innovative approaches offer a safer, more versatile alternative to traditional viral vector methods. From electroporation to chemical carriers and mechanical delivery systems, non-viral techniques provide researchers with a diverse toolkit for genetic modification across various cell types. This eBook explores the benefits of non-viral gene therapy techniques in producing the next generation of cell or gene therapy, developing cell-based assays, or driving vaccine development.
Case Study

Megan Embrey
Senior Field Applications Scientist
MaxCyte, Inc.
Empowering CDMOs: A Critical Tech Transfer Rescue
When a partner’s therapy process failed after transferring to a new CDMO, our Senior Field Applications Scientist Megan Embrey quickly arrived onsite to diagnose and fix the issue. Within days, we helped restore production and provided operator training, ensuring critical treatments reached patients in time. This case underscores our unwavering commitment: we step in wherever needed, resolve complex challenges fast, and help partners maintain momentum for life-changing therapies.
Cell & Gene Therapy
GEN News: The State of Cell and Gene Therapy Summit
Safe and Efficient Non-Viral Cell Therapy Development Using Electroporation
- Innovations in Non-Viral Gene Editing and Gene Delivery: Understand the breadth of cellular engineering beyond gene knockout and viral CAR delivery.
- Cell Therapy Case Studies: See how electroporation technology has advanced lifesaving treatments for a variety of diseases.
- Advantages of MaxCyte Electroporation: Learn why MaxCyte's platform is trusted by industry leaders. Our instruments address the dominant challenges in the industry: standardization, therapeutic complexity, regulation and time.

Ashley Strickland-Dietz, PhD
Immunology Scientist II,
MaxCyte, Inc.
Scientific Poster
Highly Efficient Engineering of Difficult-to-Transfect Immune Cells Using MaxCyte Electroporation
Cell-based therapies are advancing treatment for cancers, autoimmune disorders, and degenerative diseases. However, efficient delivery of biomolecules into immune cells like T cells, NK cells, and macrophages remains a challenge. MaxCyte’s Flow Electroporation® technology, integrated within the ExPERT™ platform, enables high-efficiency transfection of mRNA, DNA (including transposons/transposases), and CRISPR-Cas ribonucleoproteins (RNPs) with homology-directed repair (HDR) templates. These workflows achieve robust transient and stable expression of CARs and TCRs while preserving cell viability and function. Moreover, they scale seamlessly, supporting clinical and commercial-scale cell therapy manufacturing.
Scientific Brief
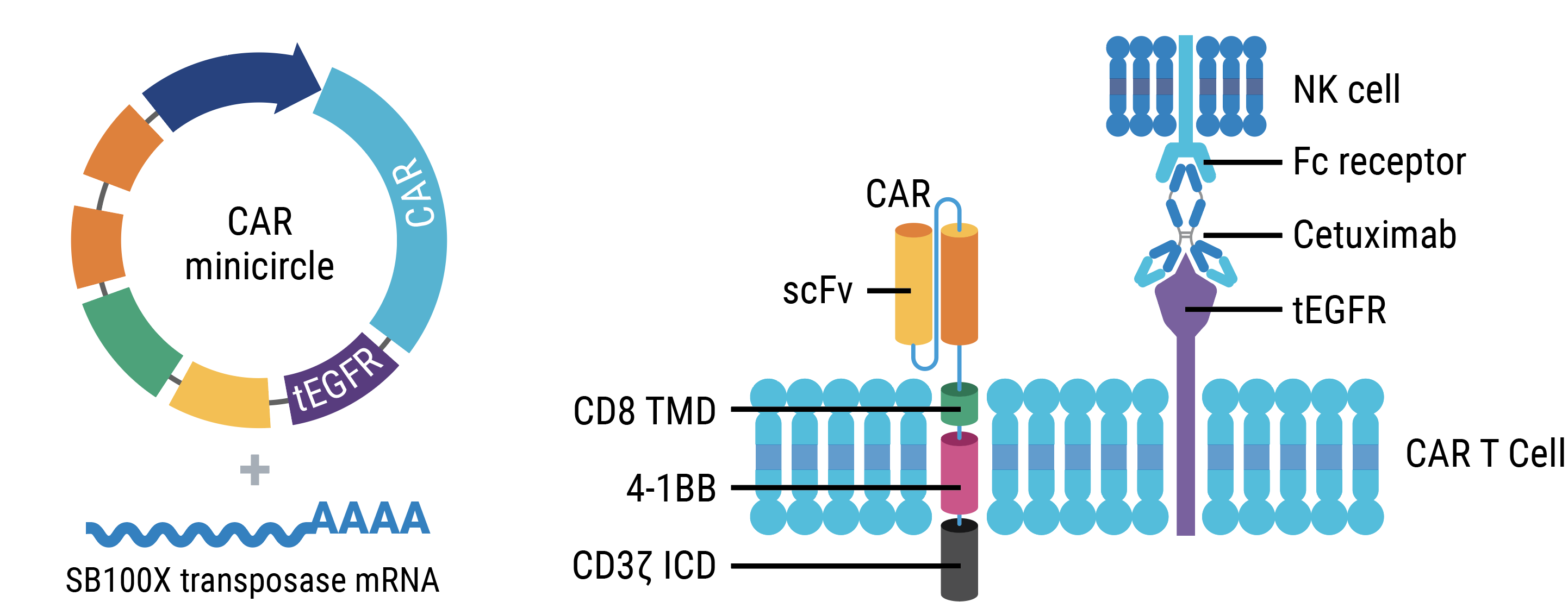
MaxCyte Enabled the Development and Rapid, Reproducible Manufacturing of TranspoCART Cells
Researchers developed a non-viral CAR T process using MaxCyte eletroporation to deliver CAR transposon minicircle DNA and Sleeping Beauty 100x transposase mRNA (SB100X) to autologous patient T cells. The manufacturing process was reproducible across two different sites and generated TranspoCART cells that prolonged survival in mouse disease model.


Dan Miller
MaxCyte Field Application Scientist
MaxCyte, Inc.
Cell & Gene Tech Expo
April 8 & 10, 2025 | 10:00 am-12:00 pm ET

Customer Testimonial
"Without MaxCyte, we would not have been able to scale our process to the level needed for our clinical program. Compared to other platforms evaluated, with MaxCyte's large-scale, closed-system electroporation process, we are able to drive down COGS, derisk the process, and have a reliable unit operation for gene editing."Director at a United States Biotech
Upcoming Events
We are excited to attend multiple conferences and host many institutional seminars and events. Find all the latest on our events page.










