When funding is limited, every decision matters. Now more than ever, researchers need to show compelling results, reduce inefficiencies, and conserve valuable time and resources.
At MaxCyte, we understand what's at stake. We help scientific teams move quickly from concept to clinic with scalable, GMP-compliant solutions and unparalleled support. We help you get it right the first time and keep advancing with confidence.
In the news
See what true partnership looks like
Not all technology providers stay with you beyond the sale. At MaxCyte®, we do.
Our Strategic Platform License (SPL) isn’t just a contract—it’s the start of a hands-on, always-on partnership designed to support you from IND preparation through global manufacturing. From accelerating timelines to reducing cash burn, we’re with you at every stage of your therapeutic development.
We’ve updated our website to make it easier to see exactly what that journey looks like—and how our SPL model enables real, measurable outcomes.

Your pathway to development
The promise of autologous and allogeneic cell therapies
In Biocompare, James Brady, PhD, MBA, SVP of Technical Applications and Customer Support at MaxCyte, shares insights on factors shaping the choice between autologous and allogeneic therapies. “It often comes down to urgency, scalability and the immune profile of the disease,” he notes, emphasizing how therapy selection depends on treatment timelines, cost and patient-specific needs.
Technology Networks with Andrew Mancini
In his recent presentation during Technology Networks' Cell and Gene Therapy 2025 Symposium, MaxCyte’s Andrew Guy Mancini, PhD, explored the rapid evolution of engineered cell therapies, and what’s next for the field. From the rise of non-viral delivery methods to the shift toward allogeneic approaches, Dr. Mancini highlighted how developers are tackling complexity, scalability and cost to bring next-generation therapies to more patients.
Case study

Investing in your success upfront
Learn how Kamau Therapeutics partnered with MaxCyte to optimize their cell engineering processes. Using MaxCyte’s GMP platform, they have advanced their lead program, nula-cel, currently in clinical development for sickle cell disease. Initially, the partnership focused on process development, culminating in a clinical collaboration announced in 2024.
"Through our collaboration with MaxCyte and the use of their proven non-viral platform, our goal is to treat or cure a range of serious genetic diseases with unmet medical needs using homology-directed repair."Matt Porteus, Co-Founder, Kamau Therapeutics, Inc.
Cell & gene therapy
UPCOMING WEBINAR
Combining CRISPR and Transposon-Based Technologies for Improved sdAb-Based CAR T Therapies
Register to discover the potential of transposon and genome editing technologies to develop next-generation, cost-effective single-domain antibody (sdAb) CAR T therapies. This webinar will explore how non-viral delivery of CAR constructs using a transposon system enhances manufacturing efficiency, improves cell viability through optimized electroporation methods, and enables the creation of universal allogeneic CAR T cells. Attendees will gain insight into how combining genome editing tools such as CRISPR-Cas9 and base editing with transposon systems supports the rapid, scalable production of therapeutic-grade CAR T cells.
Attend this webinar to:
- Understand how non-viral transposon systems can improve the efficiency and scalability of CART manufacturing.
- Learn how to integrate CRISPR-Cas9 and base editing technologies with transposon based methods to create universal allogeneic CAR T cells.
- Gain insights into pre-clinical evidence demonstrating that transposon-based and universal CAR T cells achieve comparable efficacy to conventional CAR T cells in vivo and in vitro.
- Explore robust GMP-compliant manufacturing strategies that enable cost-effective clinical translation of non-viral CAR T therapies.

Begoña Diez Cabezas
Researcher CIEMAT/CIBERER/IIS-
FJDager at CliniStem (GMP facility)

Juan Roberto Rodriguez-Madoz
Investigator Immune Therapies
Lab. Hematology Oncology-Program, Cima Universidad de Navarra

Cell & Gene Tech Expo
GMP & Regulatory Compliance: Mitigate Clinical Risk in Your Cell Therapy Development with a GMP-compliant Process from the Get-Go
At this event, Sean Menarguez shared strategies to safeguard clinical success through the early adoption of GMP-compliant processes. In his presentation, Sean covered:
- How early adoption of GMP-compliant processes can reduce downstream risks.
- A collaborative approach to accelerating development timelines.
- How MaxCyte can support your journey from research to commercialization.

Sean Menarguez
Sr. Director of Global Business
Development at MaxCyte, Inc.
Cell Therapy Solutions: High-Efficiency Cell Engineering with MaxCyte's Electroporation Platform from Concept to Clinic

Dan Miller
Field Application Scientist
at MaxCyte, Inc.
Cell-based assays
UPCOMING WEBINAR
Cell-Based Screening:
Accelerating Therapeutic Development

Therapeutics development benefits substantially from physiologically relevant assays, ideally ones using disease-relevant cells or organoids. Cell-based assays in small-molecule drug discovery have traditionally relied on stable cell lines to deliver consistent assay performance at screening scale. However, stable cell line development can be costly and time consuming. For protein or cell-based immunotherapeutic discovery, phage display is considered the gold standard, but its long selection cycles and complex downstream development can create bottlenecks. In this webinar, James Brady will discuss an advanced transfection technology that enables the development of faster, more flexible cell-based screening assays.
In this webinar, you can discover how:
- Streamlining assay development through increased speed and reproducibility
- A faster, more adaptable alternative to traditional stable cell lines, transiently transfected assay ready cells
- Accelerating antibody and cell therapy discovery with mammalian display screening

James Brady
SVP of Technical Applications
and Customer Support at
MaxCyte, Inc.
Now available on-demand
Enabling Rapid Cell-Based Assay Development with Scalable Electroporation
This on-demand presentation includes a case study on high-throughput screening of ion channel variants using automated patch clamp recordings in assay ready cells. Researchers analyzed epilepsy-associated potassium channel variants (KCNQ2), using high-throughput assays to characterize previously unstudied variants and assess their responses to a candidate therapeutic. MaxCyte’s electroporation technology enabled the creation of assay ready cells, supporting ion channel variant analysis at an unprecedented scale.
Application note
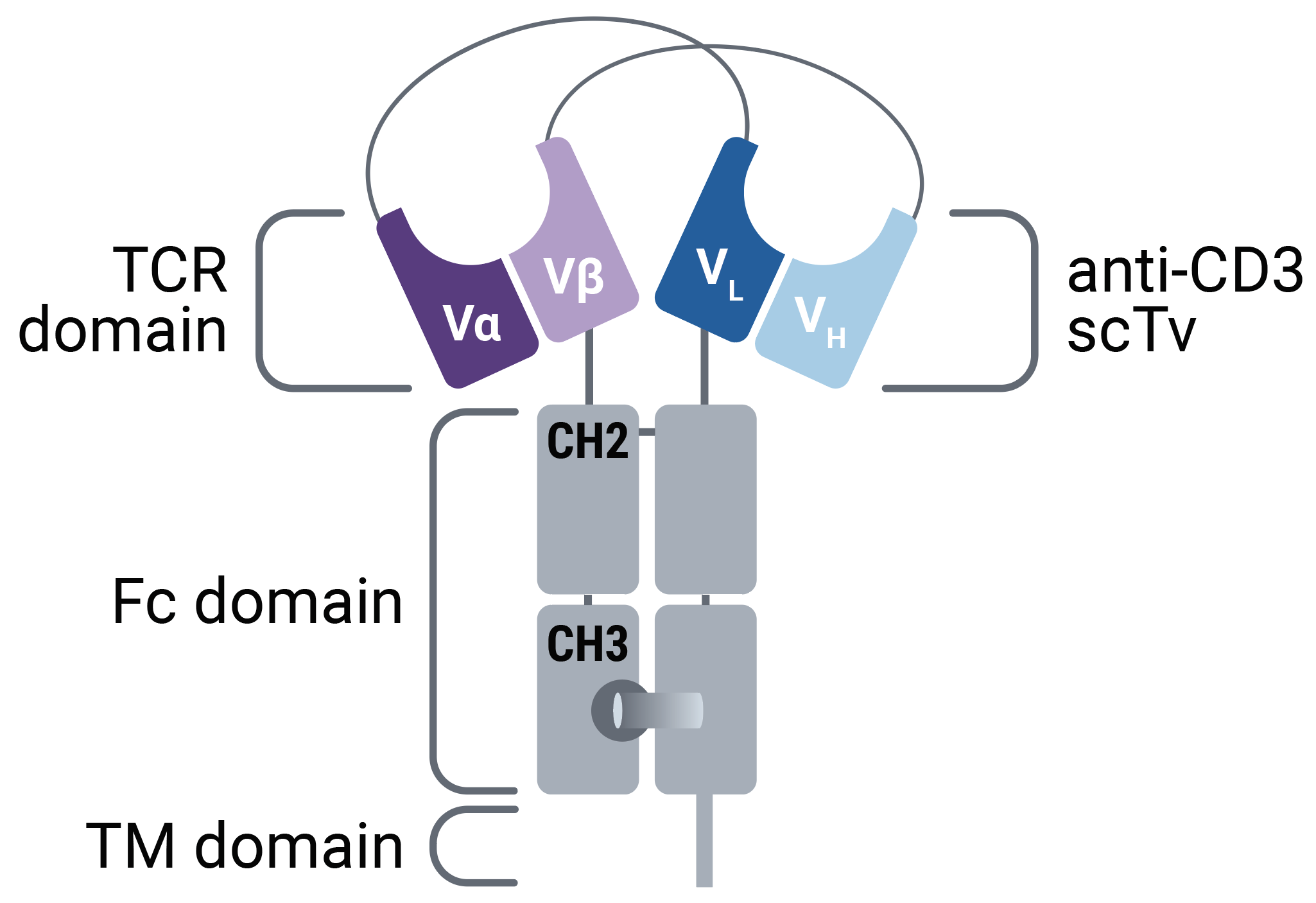
A MaxCyte-Enabled Mammalian Display Method for the Development of Enhanced Affinity TCR-Based Biologics
Researchers at the University of Stuttgart and Immatics Biotechnologies developed a mammalian display platform to select affinity-enhanced TCR sequences expressed in a proprietary bispecific T cell engaging receptor format. MaxCyte electroporation technology enabled the generation of a mammalian display library used for candidate selection and the transient expression of soluble, bispecific T cell engaging receptors for functional characterization.
Upcoming events
We are excited to attend multiple conferences and host many institutional seminars and events. Find all the latest on our events page.








