Scientific Poster
High Efficiency Complex Gene Editing of Hard-to-Transfect Primary Cells using MaxCyte Electroporation in Combination with Synthego sgRNAs
Abstract
Cell-based therapeutics using engineered primary cells hold great promise for treating a variety of diseases, including cancer and autoimmune disorders. Increasingly, researchers are using complex CRISPR gene editing strategies to develop highly engineered cell therapies with greater potency, more favorable safety profiles, and better manufacturability than previous generations of cell therapies. These CRISPR-based editing strategies often involve introducing multiple edits – including both knockouts and knockins – performed either in sequence or simultaneously to generate highly engineered cell products tailor-made for a specific disease of interest.
As the complexity of genome engineering increases, so do the challenges associated with developing efficient, scalable, and reproducible manufacturing processes. Issues arising from cell loss, low transfection efficiency, and high donor variability are often exacerbated as engineering complexity rises, increasing the risk of manufacturing failure and potentially compromising product quality.
To simplify and de-risk complex gene editing workflows, we have developed a versatile cell engineering process that combines MaxCyte electroporation with Synthego sgRNAs to enable a wide range of CRISPR-based gene editing strategies in primary human cells. Here, we show that using MaxCyte electroporation to deliver Synthego CRISPR gene editing tools (available in Research, IND-enabling, or GMP grades) as part of a cell therapy manufacturing process enables efficient and reproducible gene editing across a variety of cell engineering workflows. We show that this cell engineering strategy is suitable for use in many of the hard-to-transfect primary human cell types – including primary human T cells, NK cells, keratinocytes, and macrophages – that are increasingly being used in the development of next-generation cell therapies.
MaxCyte electroporation of Synthego sgRNAs enables efficient, scalable knockout in human T cells

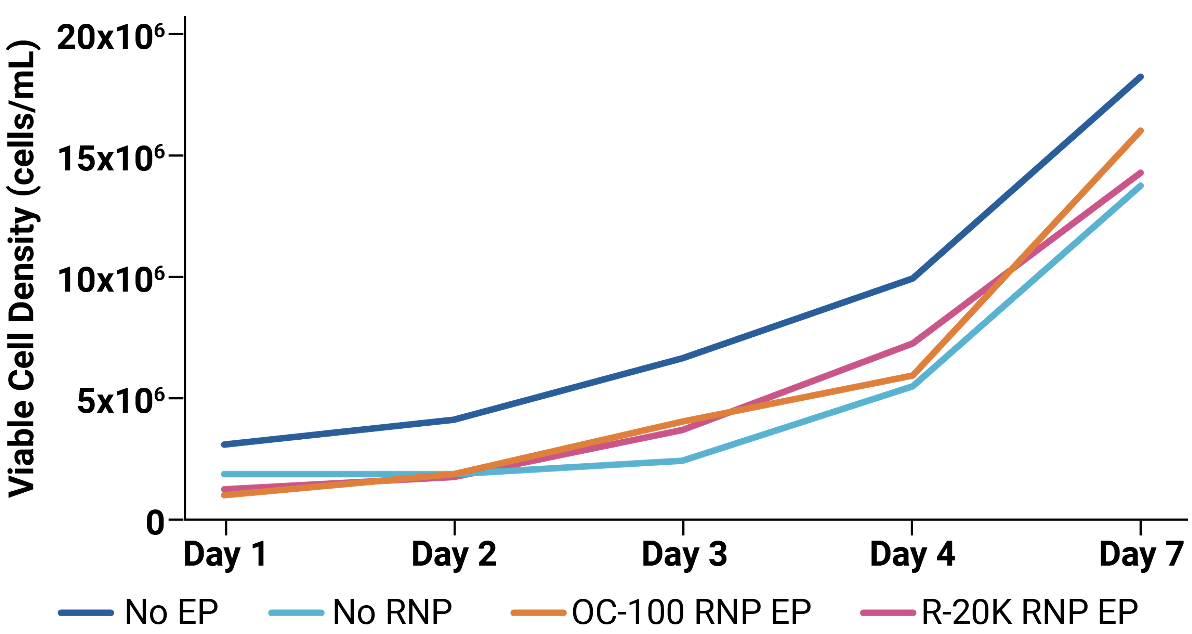
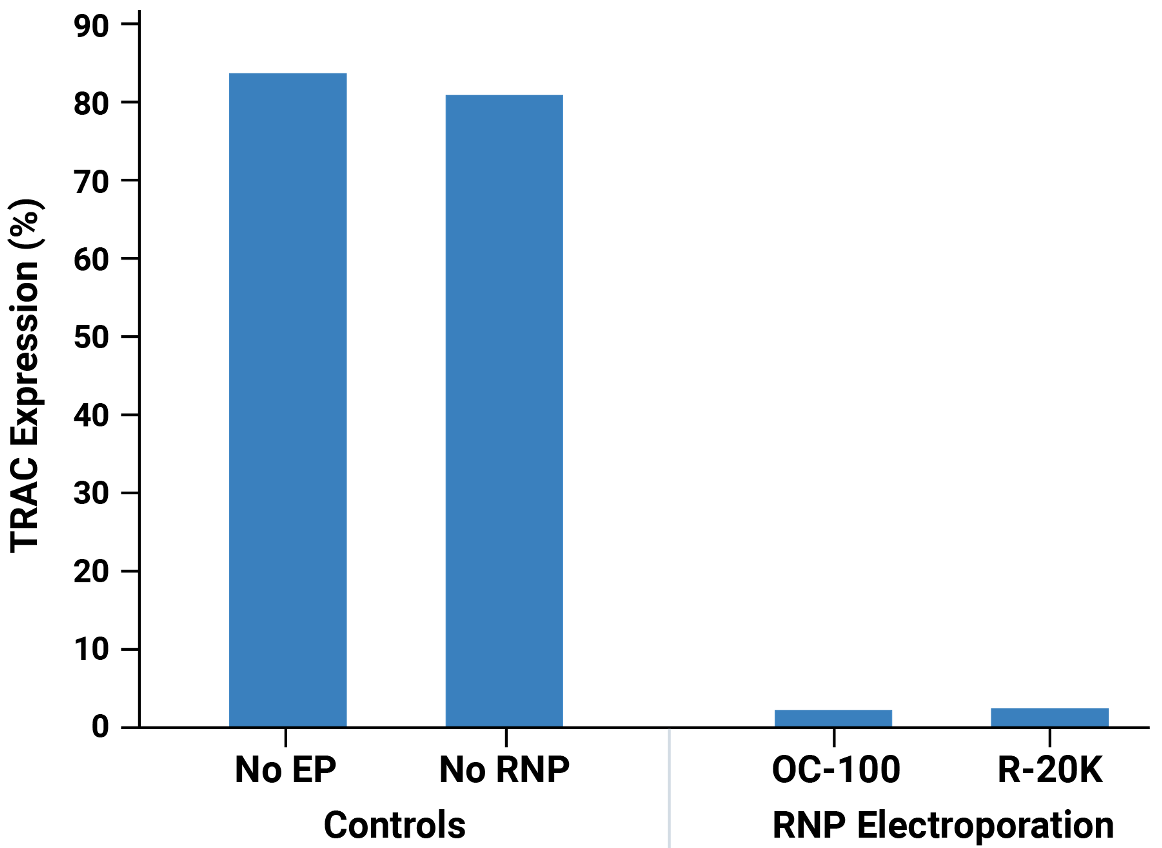
Workflow overview: Activated primary human T cells were electroporated with CRISPR RNP targeting the TRAC locus in either an OC-100 (100 μL) or R-20K (10 mL) processing assembly. Transfected cells were expanded for 3 days and TRAC and CD3 expression was assessed by flow cytometry.
A
B
C
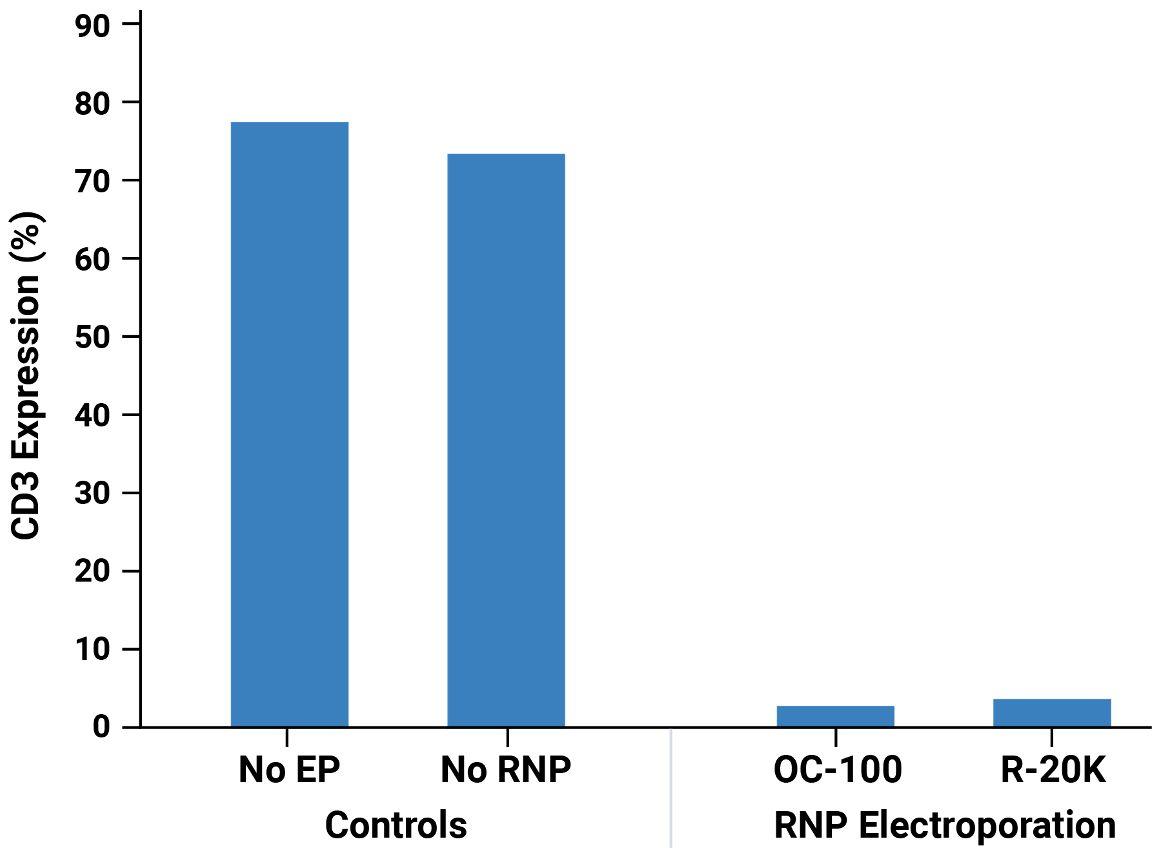
A) Viable cell density was measured on days 1, 2, 3, 4 and 7 post electroporation. Mock and RNP electroporated cells showed similar, rapid expansion as untransfected cells. TRAC B) and CD3 C) expression were effectively knocked out at day 3 post electroporation. Knockout efficiency and cell viability were consistent between cells transfected at either small scale (OC-100) or large scale (R-20K).
MaxCyte electroporation of Synthego sgRNAs enables >85% SIRP𝛼 knockout in monocyte-derived macrophages
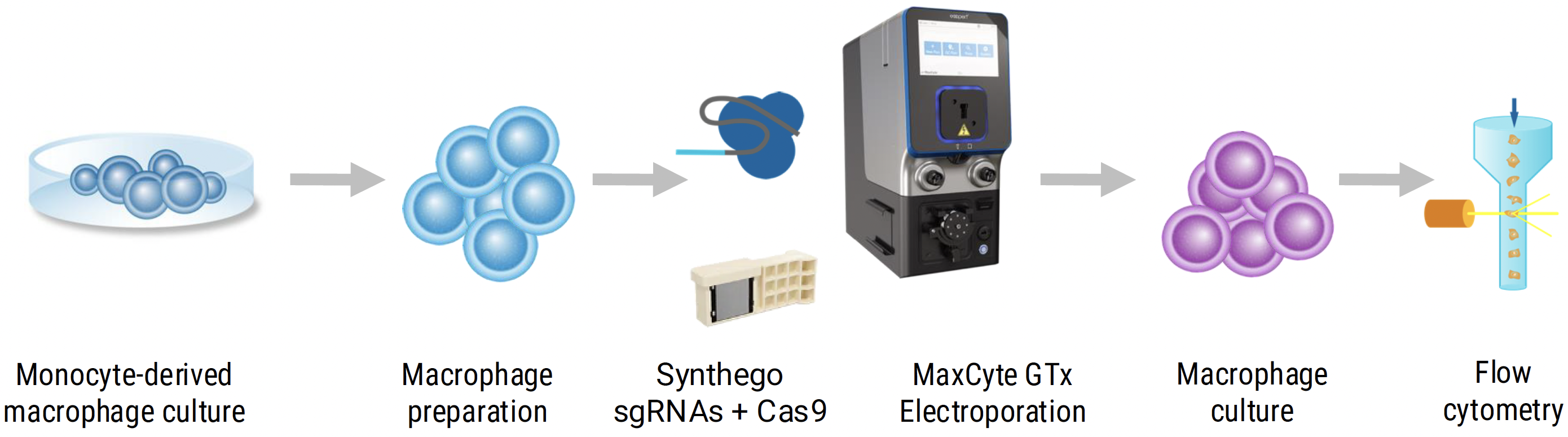
Workflow overview: Monocyte-derived macrophages were cultured for 3 days then detached, resuspended in electroporation (EP) buffer and either transfected with 2μM RNP complex (Cas9 protein and SIRP𝛼- targeting sgRNA from Synthego), mock transfected or not transfected, then returned to culture.
A
B
C
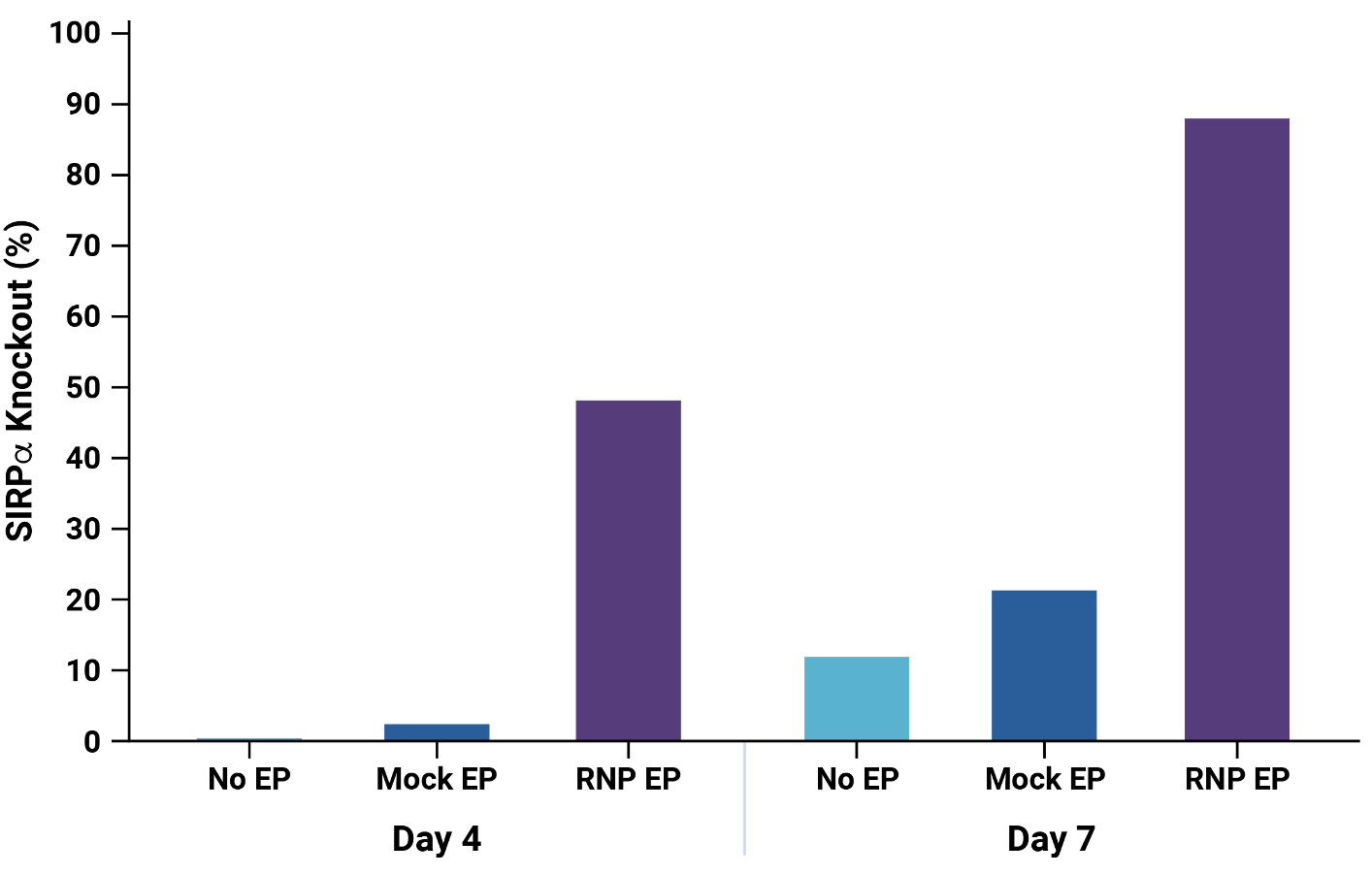
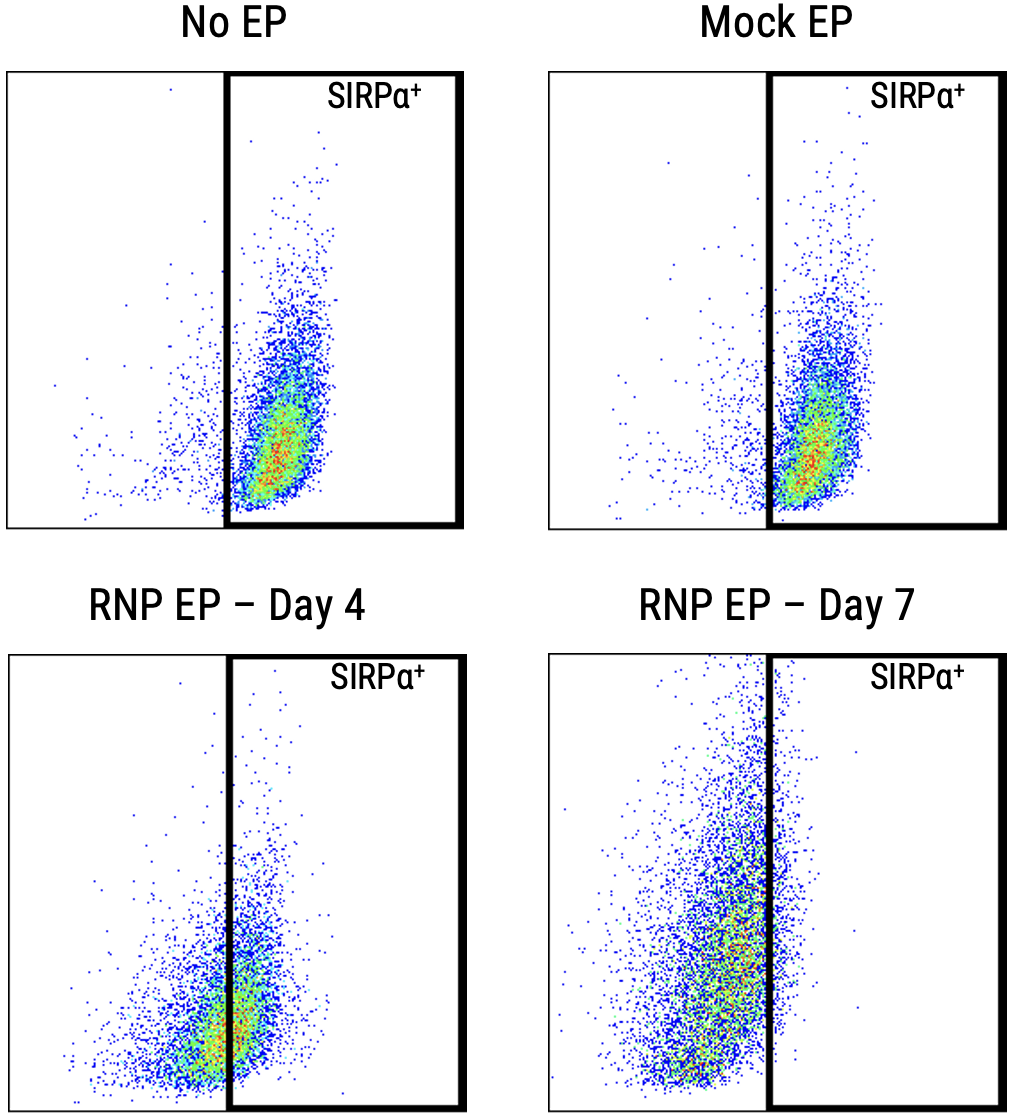
A) At day 4 and day 7 post electroporation, macrophage viability was determined. MaxCyte electroporation did not impact the macrophage population or cell viability. B) SIRP𝛼 expressed was assessed by flow cytometry at day 4 and day 7 post electroporation. C) By day 7 post electroporation, SIRP𝛼 knockout exceeded 85%.
Multiplexed CRISPR engineering of primary keratinocytes using MaxCyte electroporation in combination with Synthego sgRNAs
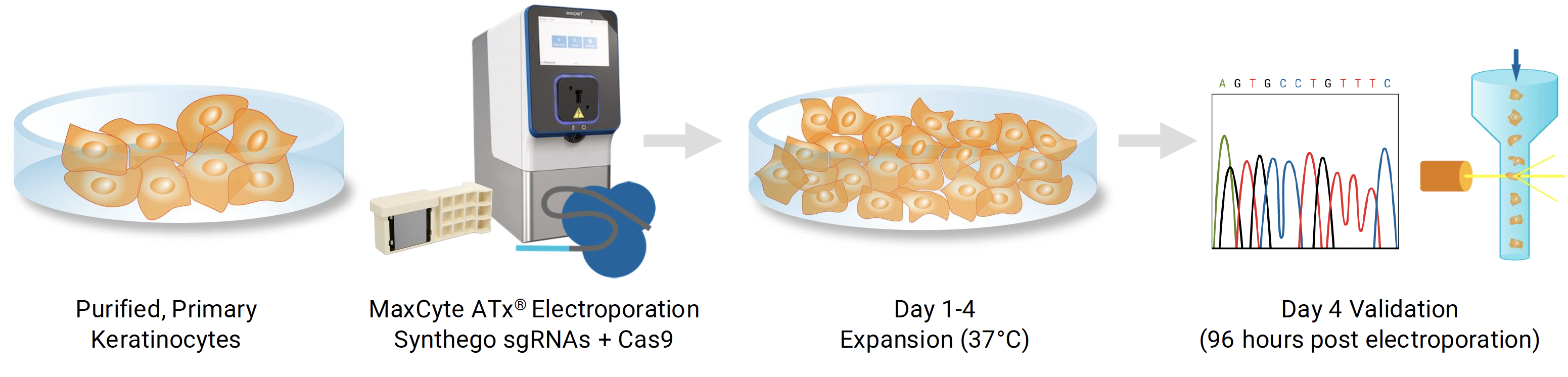
Workflow overview: MaxCyte ATx electroporation was used to deliver CRISPR/Cas9 to purified primary human keratinocytes from foreskin, tonsil and forearm skin.
A
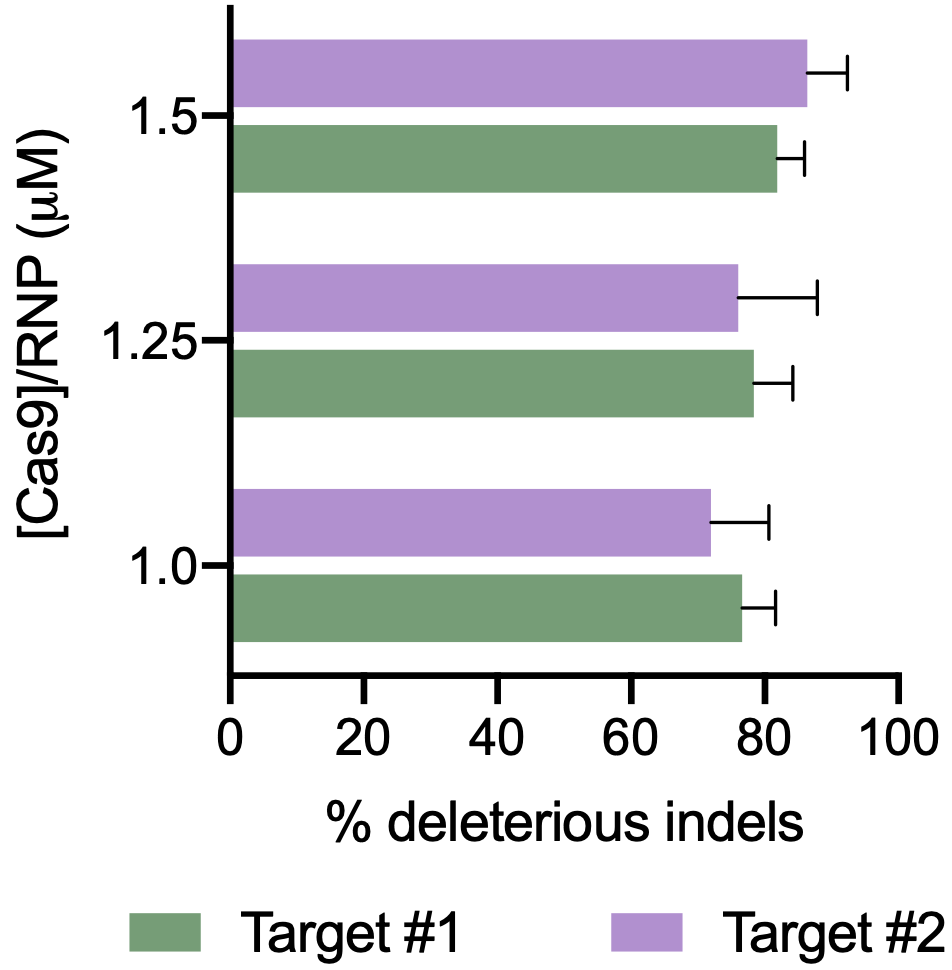
B
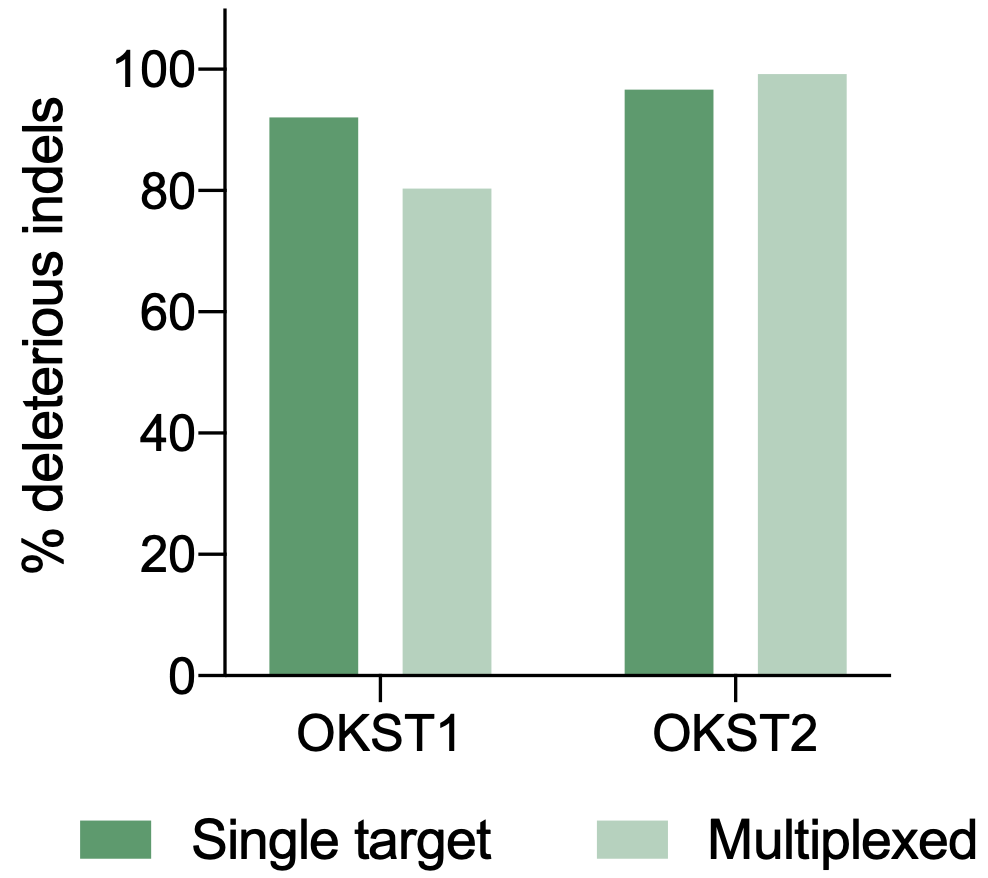
C
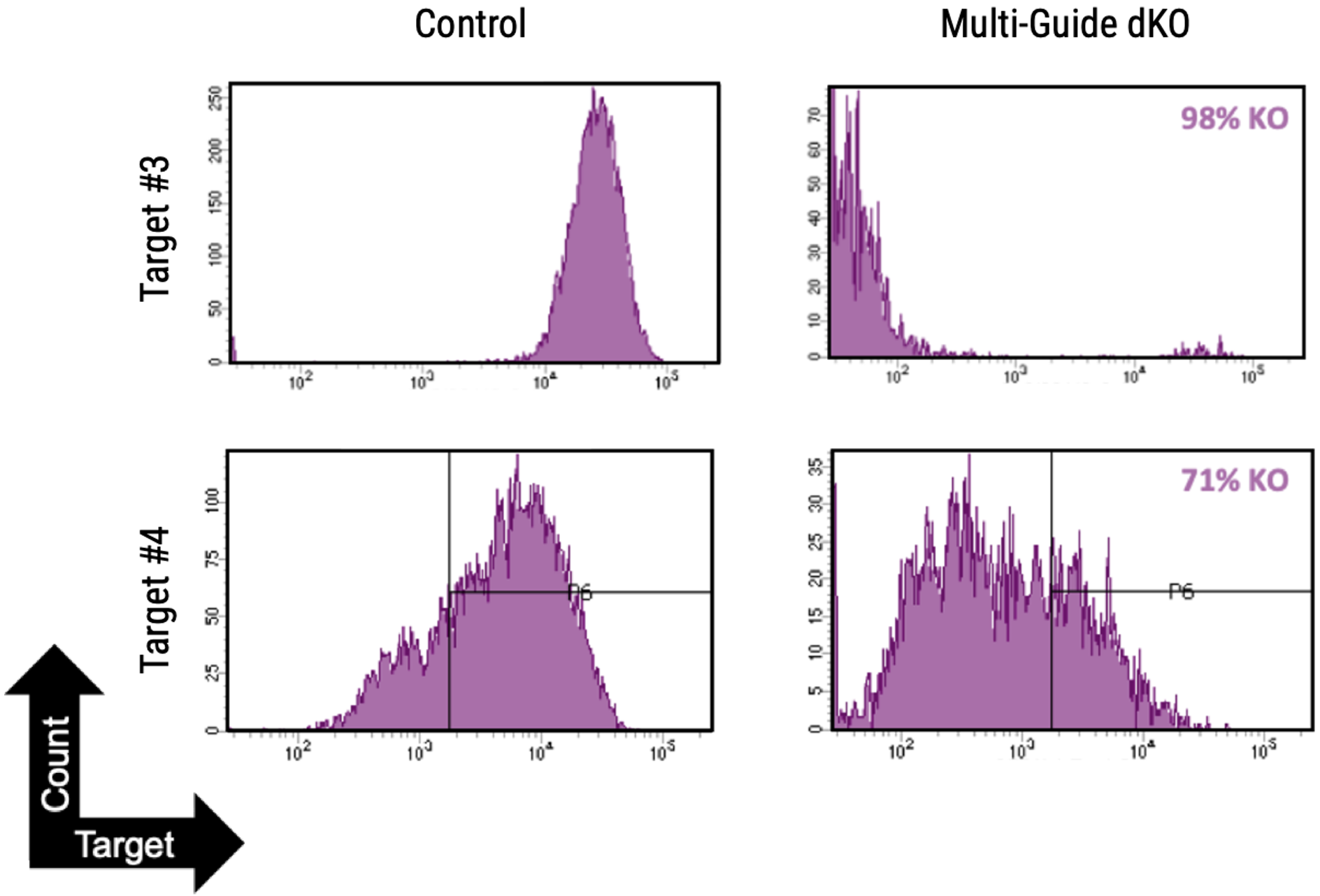
A) Percent deleterious indels measured via the TIDE assay following CRISPR RNPs electroporation in primary foreskin keratinocytes. B) Percent deleterious indels of two oral keratinocyte-specific targets (OKST1 and OKST2) measured via the TIDE analysis following electroporation of either single or dual CRISPR RNPs in primary oral keratinocytes. C) Flow cytometry analysis of two cell therapy targets following electroporation of 6 CRISPR RNPs using the Synthego multi-guide gene knockout kit in primary forearm-derived skin keratinocytes.
Data produced in collaboration with Tward lab at University of California, San Francisco. Reproduced with permission.
CRISPR RNP and ssDNA co-electroporation in activated T cells for efficient CAR knock-in
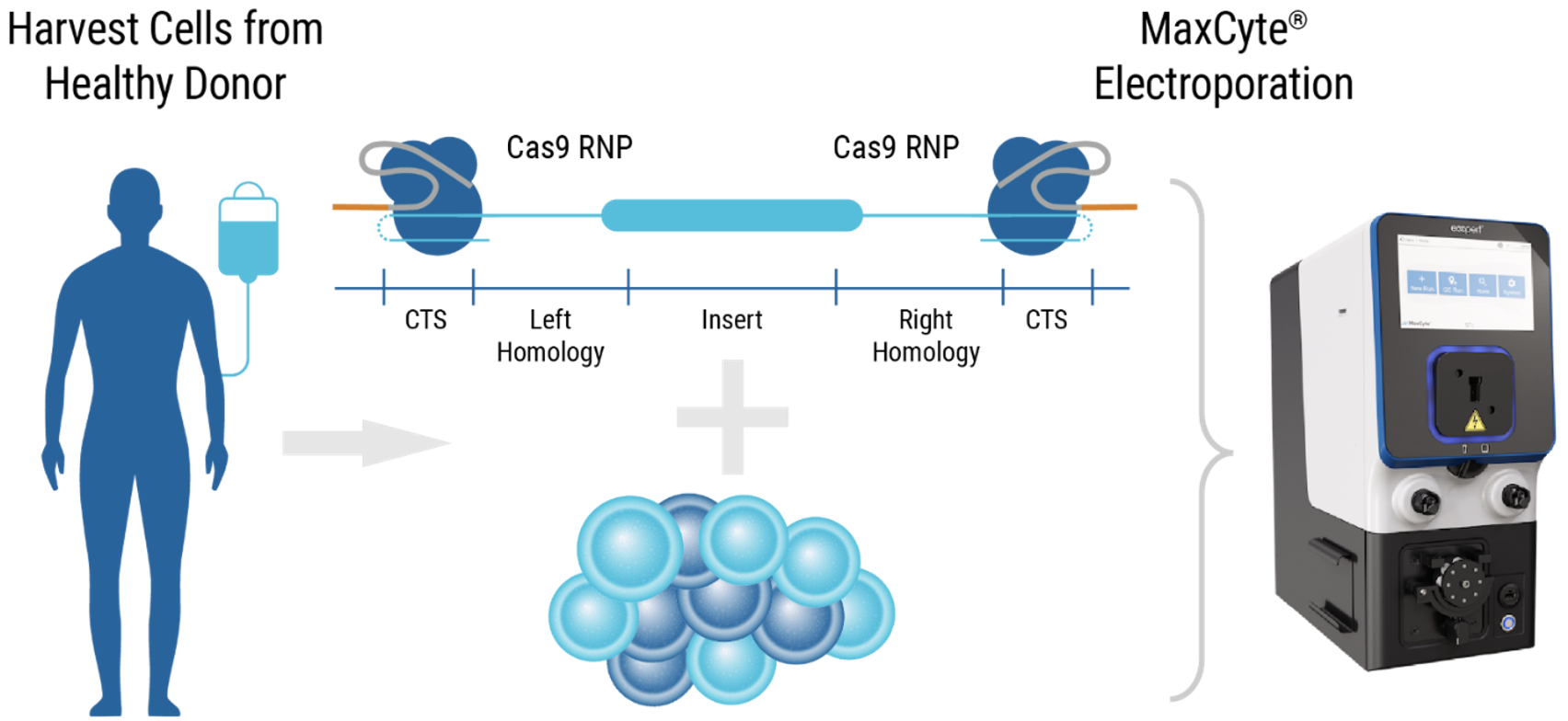
Workflow overview: ExPERT GTx electroporation was used to deliver CRISPR RNP + ssCTS template to the TRAC locus of activated T cells.
A

B
C
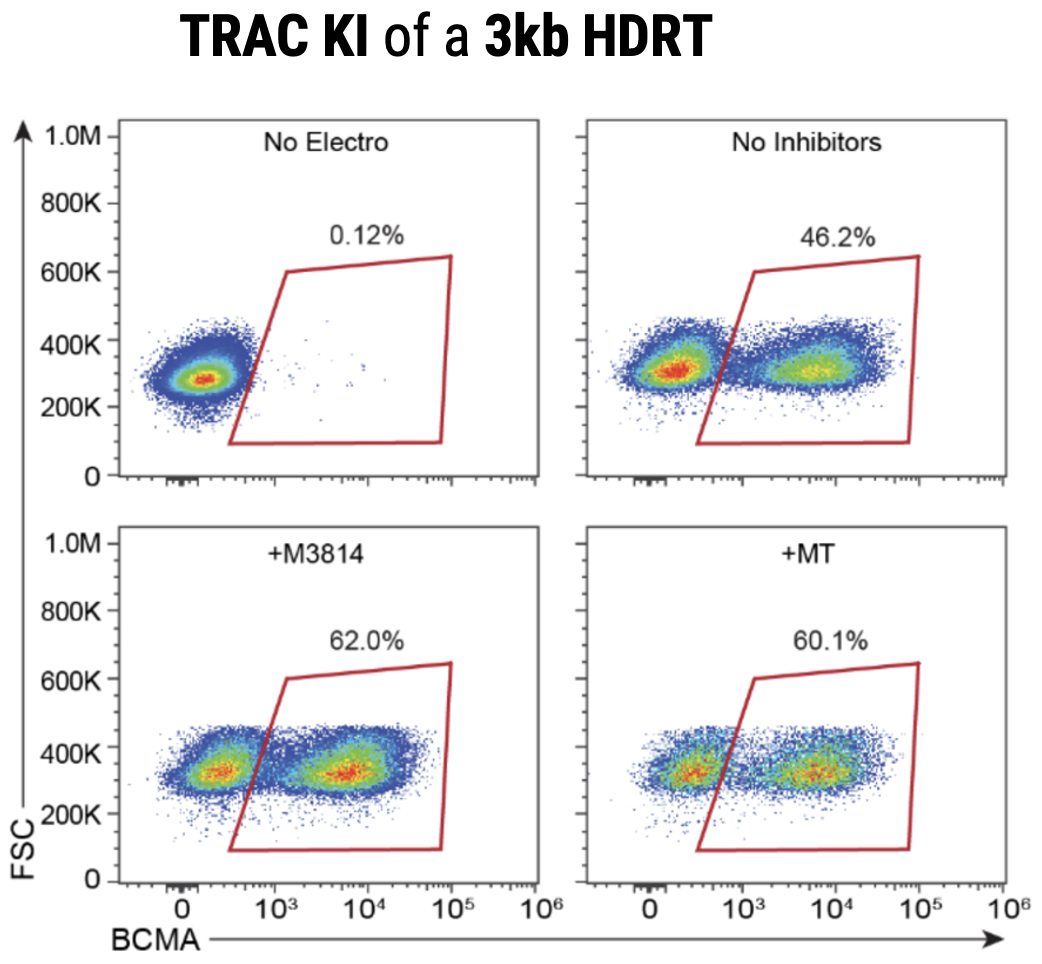
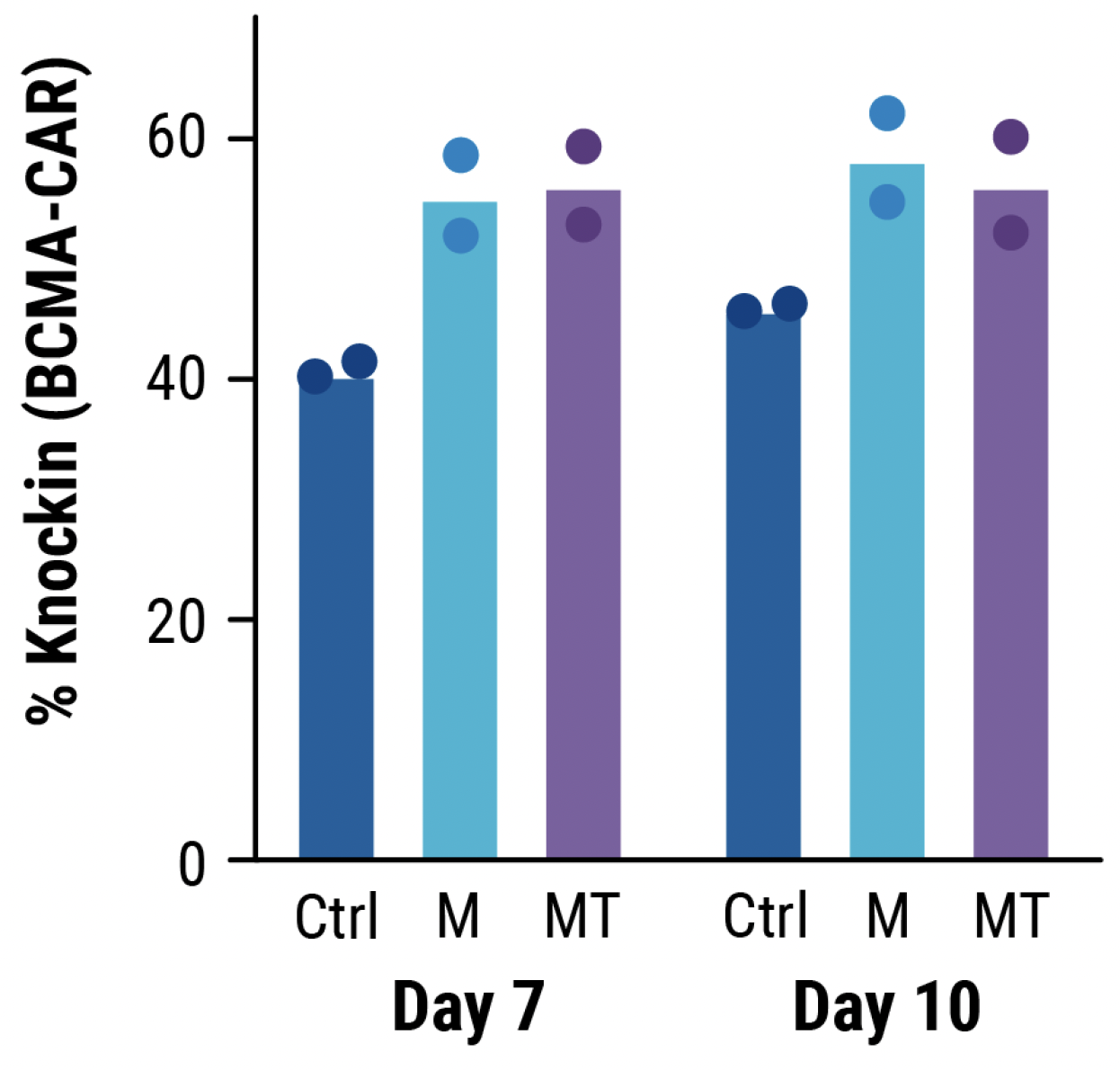
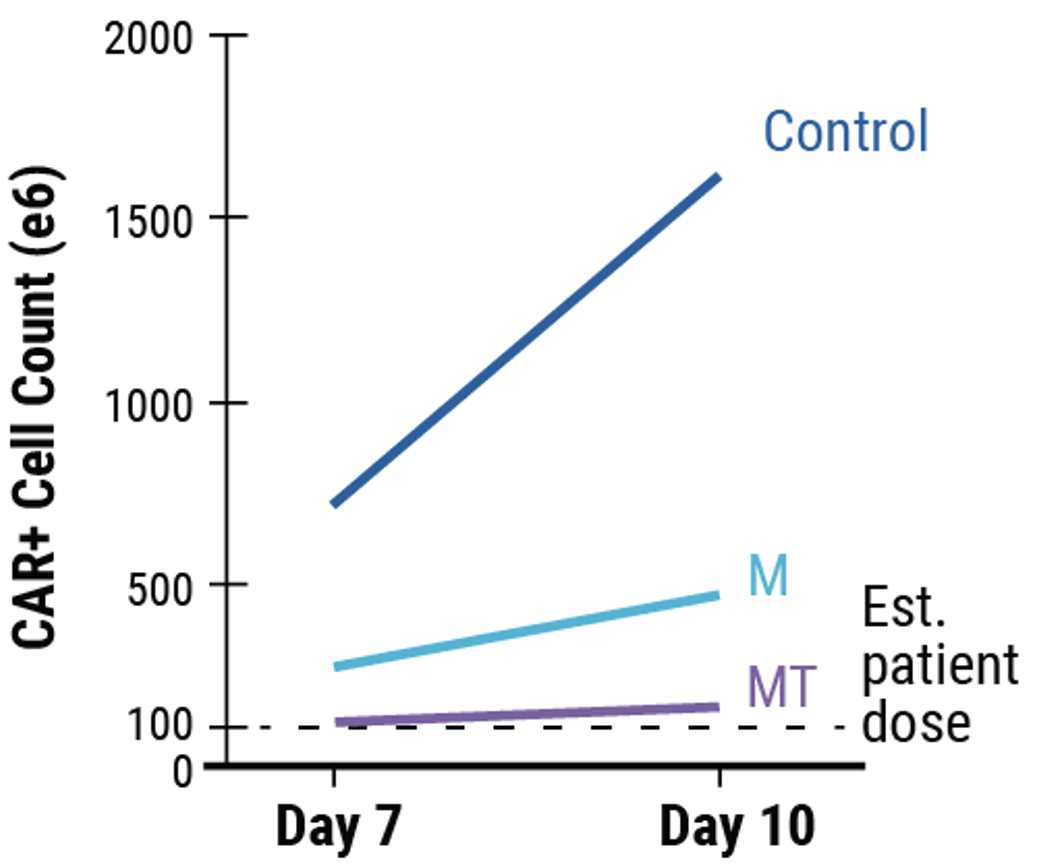
A) KI efficiencies of BCMA-CAR on day 7 and 10; Ctrl - no inhibitor, M - M3814M DNA-PK inhibitor and MT - M + histone deacetylase class I/II Inhibitor. Dots represent the KI rates for each donor. B) The total number of CAR+ T cells on days 7 and 10 of the cGMP manufacturing protocol. Dotted line - estimated patient dose of 1x108 cells. C) Flow plots of BCMA-CAR KI efficiencies for each condition on day 10.
D
E
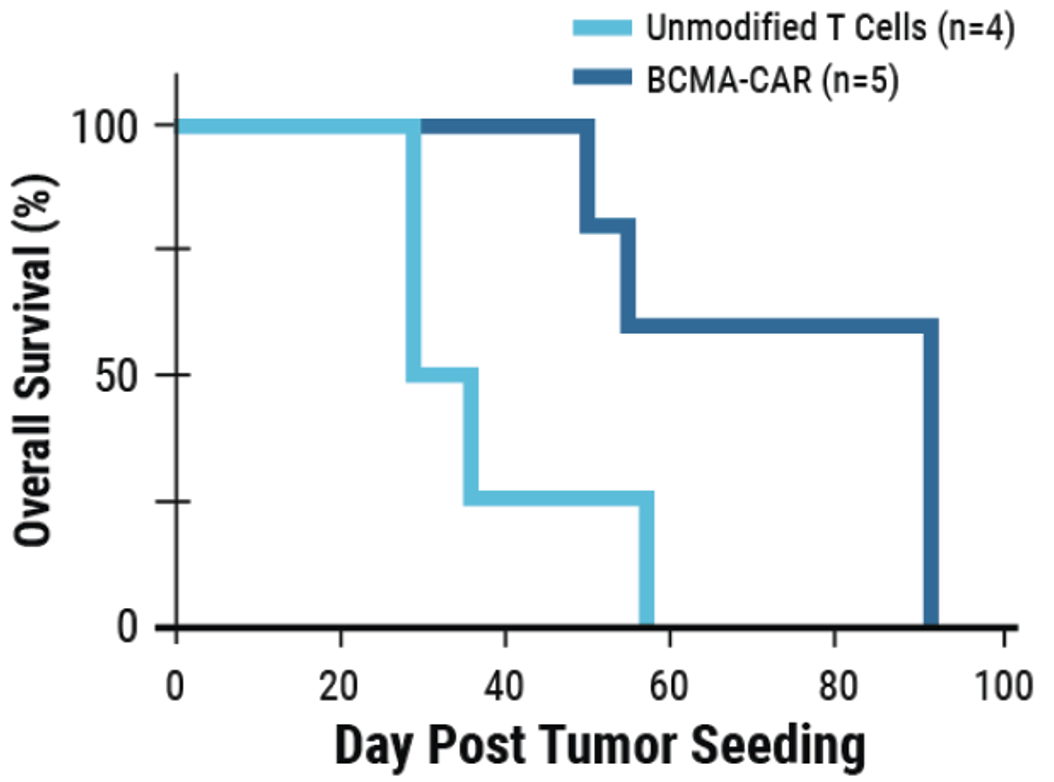
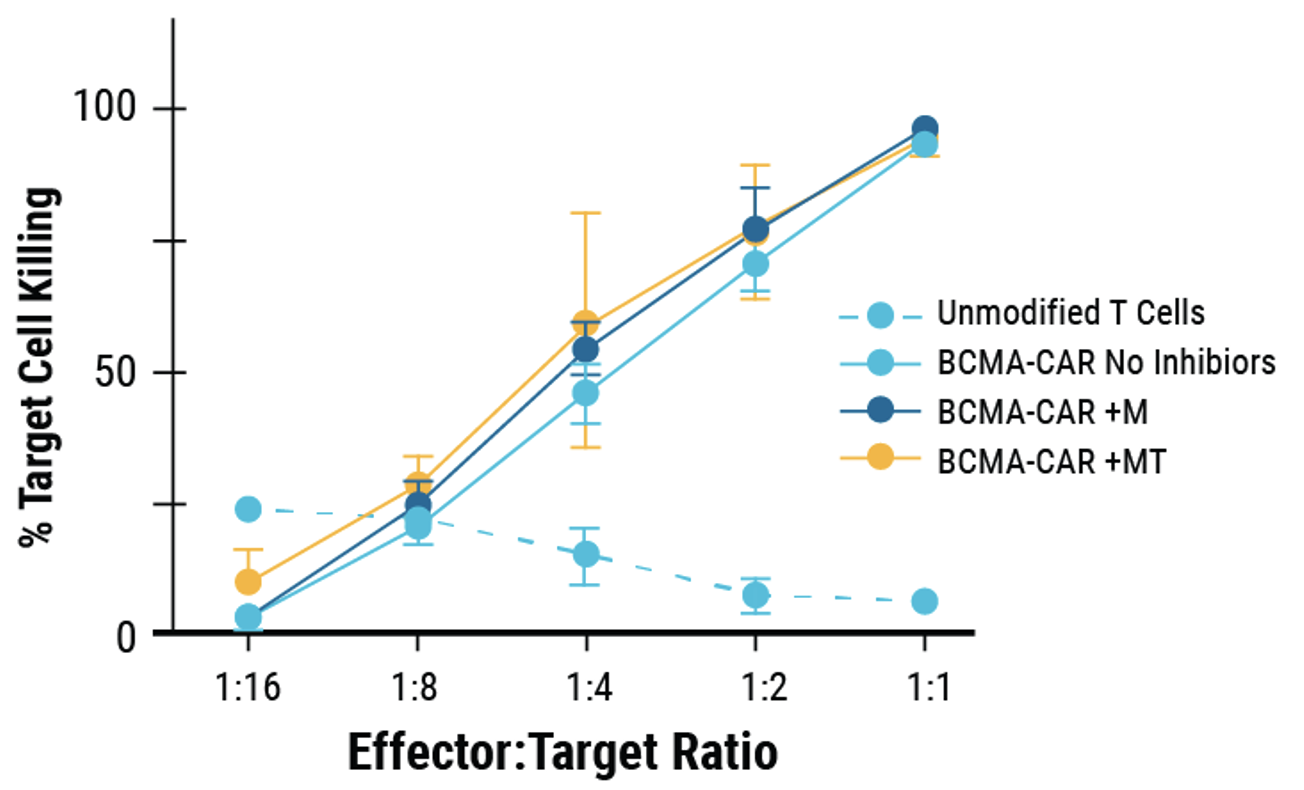
D) BCMA-expressing multiple myeloma cell lines were effectively targeted and killed by engineered CAR T cells; Effector:Target, 1:1. E) Overall survival was improved in tumor-bearing mice when treated with engineered CAR T cells or unmodified T cells from the same donor.
This content was adapted from Shy BR., Nat Biotechnol. 2023 Apr;41(4):521-531. doi: 10.1038/s41587-022-01418-8. PMID: 36008610.
MaxCyte and Synthego enable reproducible cell engineering of primary human NK cells
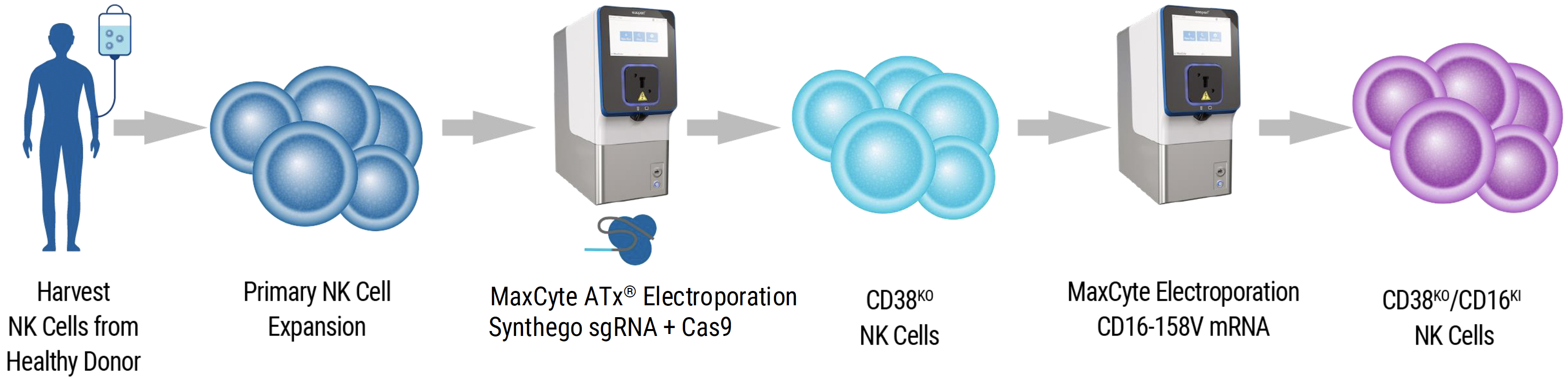
Workflow overview: MaxCyte ATx electroporation was used to deliver CRISPR/Cas9 RNPs containing Synthego sgRNAs to expanded primary human NK cells. After an additional 7 days in culture, CD38KO NK cells were electroporated with CD16-158V mRNA to create CD38KO/CD16KI NK cells.
A
B
C
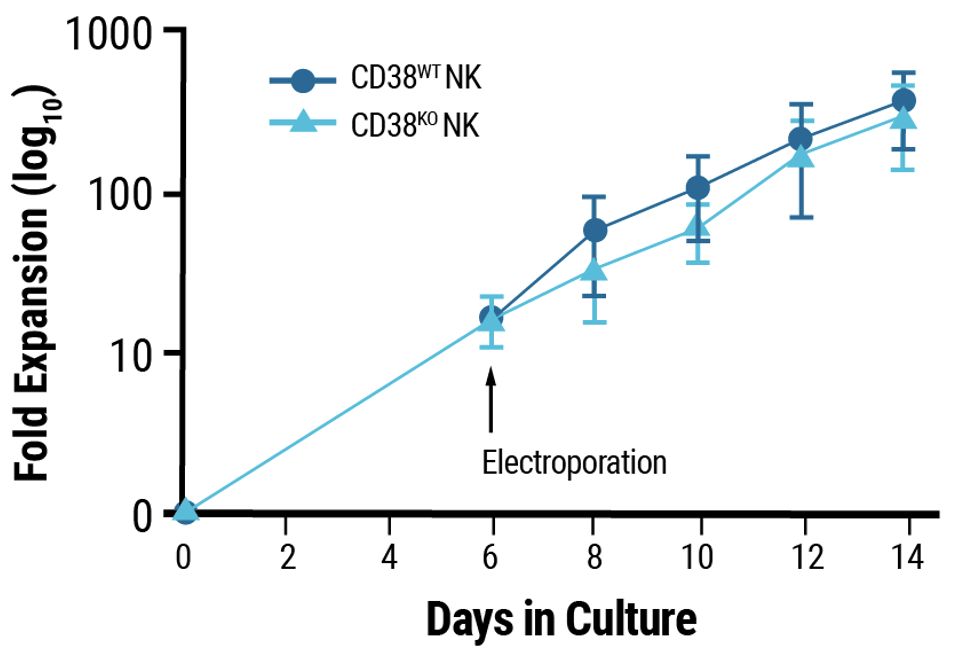
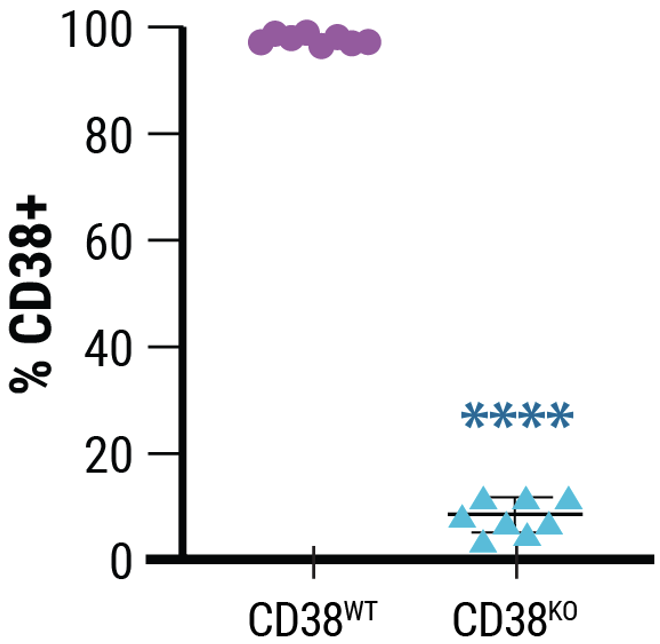
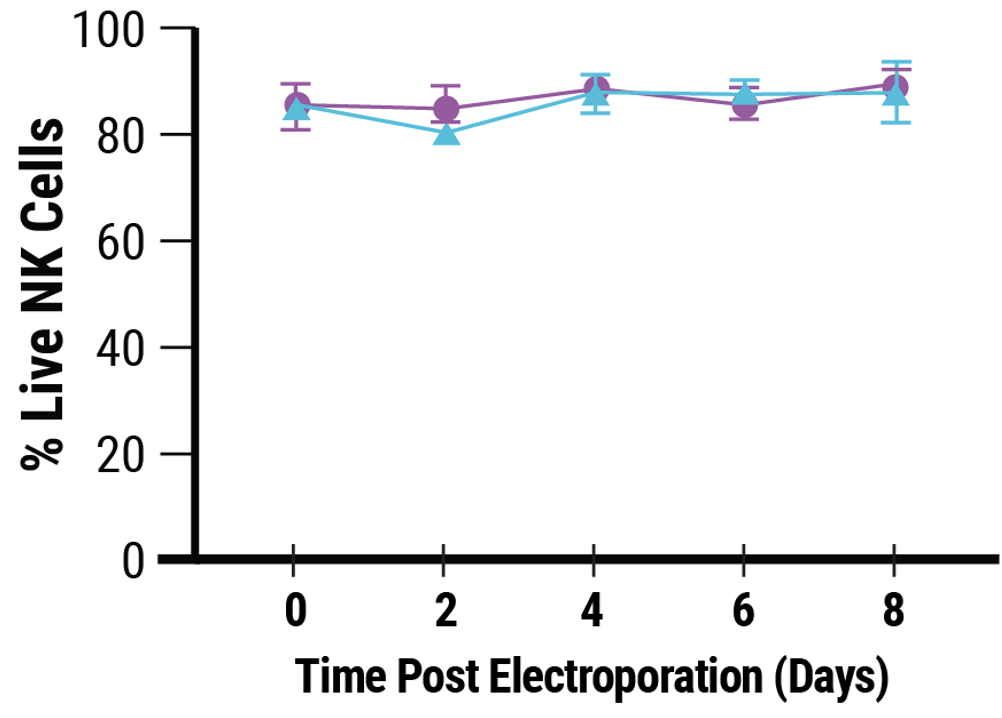
A) CD38 expression was knocked out in 92% of electroporated cells. B) Viability remained high following MaxCyte electroporation of a Synthego sgRNA targeting CD38. C) Electroporation and CD38 knockout did not impair NK cell proliferation.
D
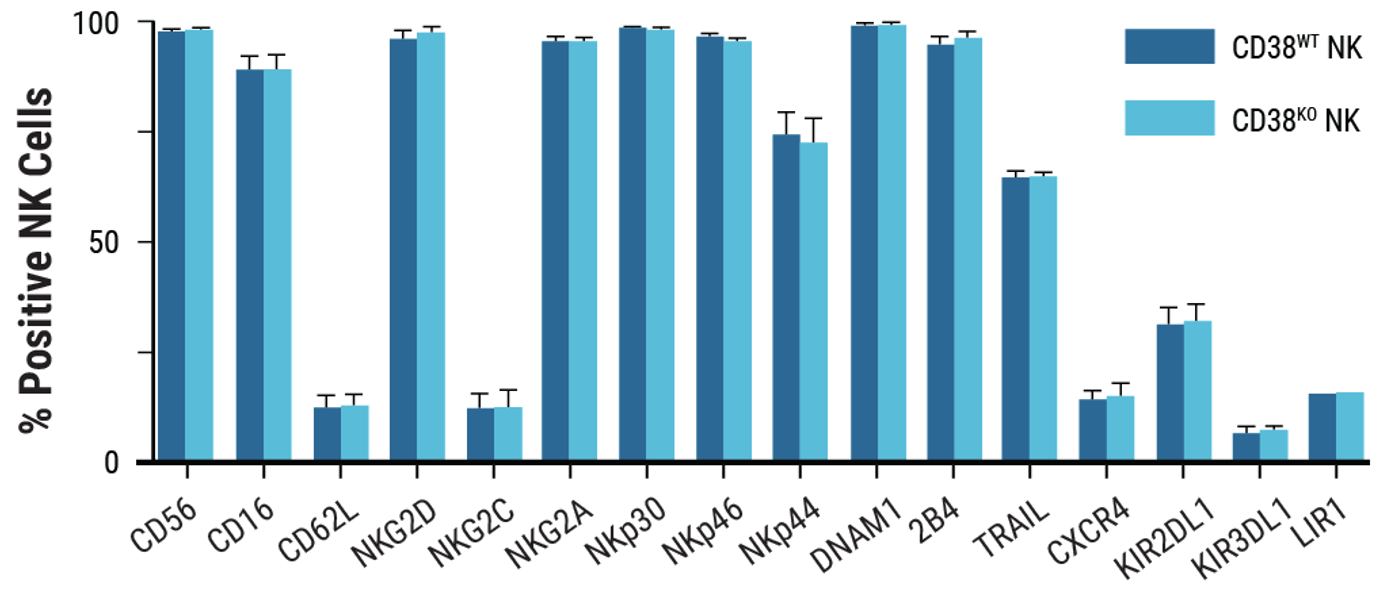
E
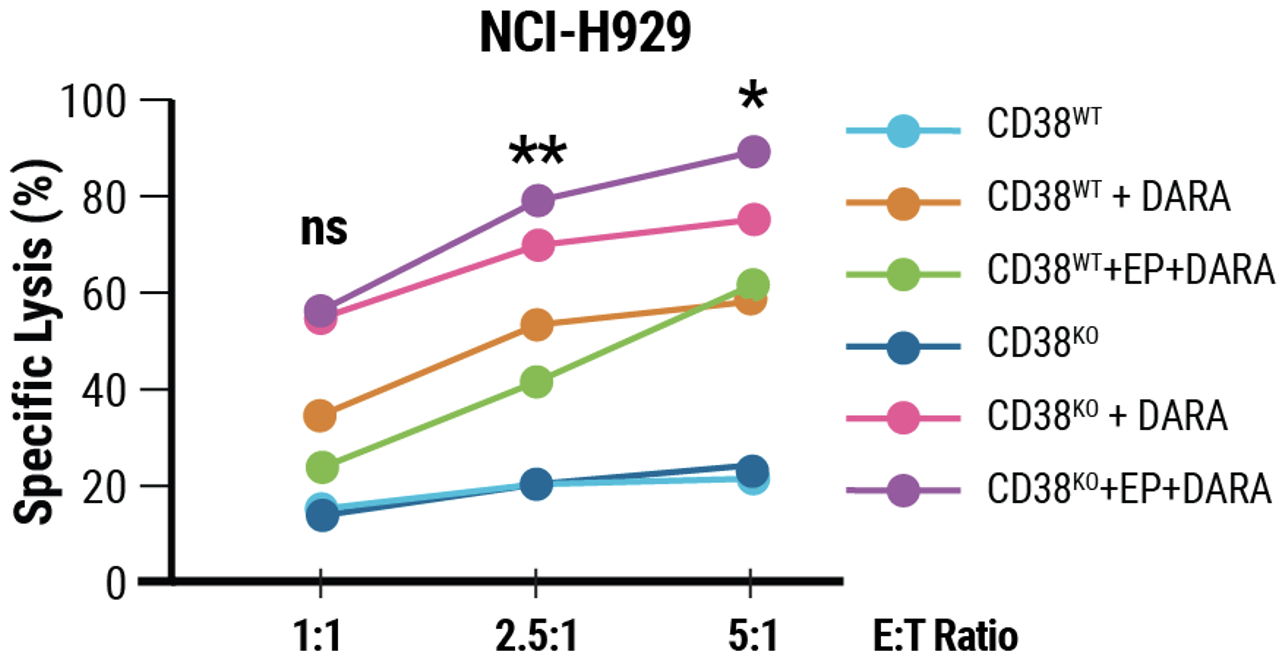
D) CD38KO and CD38WT NK cells showed identical surface marker expression following incubation with target cells. E) CD38KO/CD16KI NK cells showed enhanced lytic activity towards CD38+ NCI-H929 myeloma cells.
This content was adapted from Joseph Clara et al. J Immunother Cancer. (2022) under the Creative Commons license Attribution 4.0 International (CC BY 4.0) doi: 10.1136/jitc-2021-003804. PMID: 35135865; PMCID: PMC8830298.
Summary
MaxCyte electroporation delivers Synthego CRISPR gene editing tools (available in Research, IND-enabling, or GMP grades) at any scale for efficient and reproducible complex gene editing in a variety of primary human cells.
-
- MaxCyte electroporation of Synthego sgRNAs delivers greater than 85% gene knockdown in primary human macrophages with no effect on cell viability.
- Multiplexed delivery of multiple Synthego sgRNAs by MaxCyte electroporation resulting in efficient multi-target CRISPR genome editing in human keratinocytes from different primary sources.
- MaxCyte and Synthego enable the generation of enhanced functionality CD38KO/CD16HA NK cells by sequential CRISPR editing and mRNA delivery in expanded primary human NK cells.
- MaxCyte and Synthego integrate into a GMP-compatible process to aid rapid clinical translation of highly efficient non-viral CAR T cell manufacturing via CRISPR-mediated knockin.







